Contact Us
US: +1 512 898-9222
SG: +65 3138-4148
EMAIL: VietNam@asiaactual.com
Grouping requirements in Vietnam are guided by Appendix II of Circular 39/2016 released in October 2016. Grouping allows for a simplification of the application process, which in turn, is more cost effective and time saving for the manufacturer. To be grouped together, the products will need to adhere to the specific rules to belong to certain categories.
The different grouping categories are as follows:
Three general principles generally apply to each category:
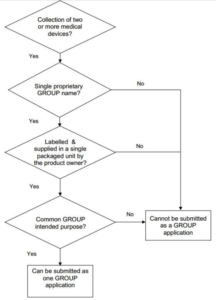
Below are excerpts from Circular 39/2016 outlining the permissible grouping variations and relevant definitions. More information on grouping requirements in Vietnam, including decision flow charts, please see the Circular.
A single medical device is a medical device from a product owner identified by a proprietary name or brand name with a specific intended purpose and sold as a distinct packaged entity and that cannot be assigned into a family, IVD test kit, system, IVD cluster or group.
Examples:
A medical device Family is a collection of medical devices and each medical device family member:
Examples of permissible Family variants can be found in Appendix II of Circular 39/2016.
A medical device System comprises of a number of medical devices and/or accessories that are:
from the same product owner;
An IVD Test Kit is an in vitro diagnostic (IVD) device that consists of reagents or articles that are:
An IVD Cluster comprises of a number of in vitro diagnostic reagents or articles that are:
A list of common test methodologies and IVD cluster categories can be found in Table 2 of Appendix II of Circular 39/2016.

David Vo, General Manger, Asia Actual Vietnam
David’s Regulatory Hint
“Grouping guidance is closely aligned with GHTF guidance but one main difference is that accessories sold separately will either need to be registered separately or not registered at all.”
US: +1 512 898-9222
SG: +65 3138-4148
EMAIL: VietNam@asiaactual.com
Who can submit submit applications in Vietnam?
A local entity must submit applications, hold the license, and correspond with the Ministry of Health. License Holders can then authorize an unlimited number of importers or distributors.
How are products classified in Vietnam?
Medical devices are classified into 4 categories according to GHTF guidelines; from low-risk Class A to high-risk Class D.
How long does it take to register my medical device in Vietnam?
Class A and B are immediately listed and Class C and D applications require 30 to 60 days.
How much does it cost to register my device in Vietnam?
Class A fees are about US$45 and Class D are about US$220.
How long are medical device licenses valid in Vietnam?
Medical device licenses do not expire in Vietnam.
Does the license holder need to be a part of the importing process?
No, license holders can authorize multiple importers to import under one license.
Is there an expedited review pathway?
Yes, Class C and D products approved by a reference country will be reviewed in half the time. Reference Countries in Vietnam include: USA, Canada, Europe (incl. UK and Switzerland), Australia, Japan, China, and South Korea.
Is ISO 13485 required for medical devices in Vietnam?
Yes, ISO 13485 is required for registration in Vietnam.
Is Home Country approval required?
No, there are no requirements for home country approval. Reference country approval will significantly improve the review time.
Do documents need to be translated to Vietnamese as part of the application process?
Yes, some application documents will need to be translated including the Instructions for Use (IFU) and the labeling.
Contact Asia Actual for a free consultation discussing the potential for your medical device or IVD in the Vietnamese market. Asia Actual is available to help navigate the complex medical device registration requirements and regulatory pathway for medical device and IVD distribution in Vietnam. Please let us know if we can help with medical device grouping in Vietnam.
実際の亞洲
เอเชีย แอคชวล
एशिया वास्तविक
실제 아시아
515 Congress Avenue, Suite 2100
Austin, TX 78701
+1 512 898-9222
Contact Us
Privacy Policy
116 Changi Road, #04-05
