Contact Us
US: +1 512 898-9222
SG: +65 8800-3197
EMAIL: inquiry@asiaactual.com
Balancing regulatory workload spikes against fixed internal resources? Consider contracting Asia Actual for:
Asia Actual’s regulatory managers are amongst the most experienced in their market and available for discussing outsourcing solutions with your company. For certain cases, we can also offer options for Full Time Employment (FTE) through our external network of regulatory experts all over the world. Click here to meet our globe-spanning team.
If you are looking to hire regulatory staff to your team, consider Asia Actual outsourcing solutions. Our staff has decades of experience and hundreds of registrations approved.
/
Currently, the effects of the European MDR (Medical Devices Regulation) are being felt worldwide. No one understands more than us the importance of following the most recent developments and changes in medical device regulation around the globe.
At Asia Actual, we employ experienced, bilingual regulatory and commercial professionals located in countries across South Asia. This keeps us uniquely in-tune with regulatory trends worldwide and fully equipped to offer responsive outsourcing solutions. With leading professionals who have decades of experience, your company will be in safe hands throughout the entire registration process.
Asia Actual can provide a variety of support: from regulatory experts to high-level review to project management.
Many manufacturers are now facing an increase in regulatory requirements, whether from MDR updates or new registration requirements in India, Thailand, Vietnam, and the Philippines. Asia Actual’s experienced staff can help step in and provide seamless additional regulatory support on your behalf or to your local staff.
Thailand is a recent example of how critical it is to keep up with regulatory trends, as their shifting regulations have increased the steps necessary for a manufacturer to get their medical device registered. Previously, only two regulatory documents were required and several products could be registered together under one license.
Now, under the latest rules, all technical documents (CSDT) must be submitted for each product family, making a lengthier, more complicated process.
India is another example of country’s registration rules being updated more quickly than manufacturers can keep up with. All non-notified Class A and B medical devices had to be registered by October 1st, 2022 or face market interruption—a grace period has been announced.
Now, there is a new deadline for previously non-notified Class C and D devices to be registered by October 1st, 2023. Keeping up to date with current regulatory updates can be difficult—but not with the regulatory professionals at Asia Actual on your side.
The Philippines is currently transitioning to requiring Certificates of Medical Device Registration (CMDR) for all products. At the moment, many products currently only require Certificates of Medical Device Notification (CMDN) but, as part of this transition, will need to soon register for a CMDR once their initial CMDN expires after 1 to 2 years.
Given the documentation requirements for CMDRs are significantly different from (and more extensive than) CMDNs, many manufactures will find themselves with a significant influx of short term regulatory needs. Asia Actual’s experienced consultants can step in and help successfully make the transition without any market interruption.
With the job market more hectic than ever, it can be hard to find and retain quality staff. Let us help you by outsourcing your RA work to our experienced consultants located in countries throughout Asia. Support can include:
Most importantly, you will be immediately welcomed into an experienced network of professionals who intimately understand the ever-changing world of medical device regulation, ensuring a smooth and straightforward process.
Unlike other companies, at Asia Actual we have the capacity to offer RA support in a variety of different markets, including Singapore, Malaysia, Vietnam, the Philippines, Thailand, Indonesia, Australia, India, China, Hong Kong, Japan, South Korea, and Taiwan.
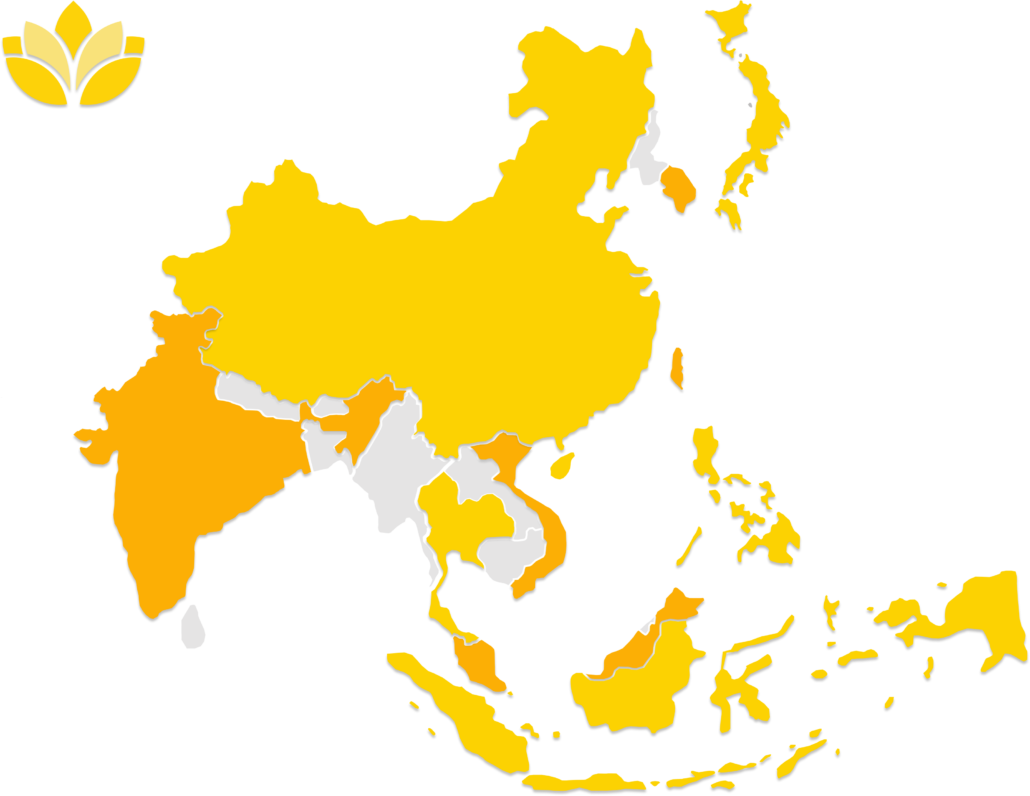
Countries where Asia Actual provides RA support
Our work in all of these countries can be covered under one agreement, instead of having to contract a different company for each region. This ensures the process remains under one umbrella and you don’t have to go through the hassle of qualifying and managing multiple entities at the same time.
In the event your company does not have offices set-up in a particular market and wishes to rectify this, we are able to augment your resources or plug holes in your current set-up. Additionally, we can perform training for your employees and other ancillary duties to ensure your company’s success.
We understand the need for post-market surveillance to mitigate any complaints or adverse events that may arise around your product, which is an additional service we can perform on your company’s behalf.
With our almost 10-years of professional, transparent regulatory support and commitment to getting our client’s products registered around the world, it’s clear when your company needs to ensure rapid market access for your products, turn to Asia Actual.

Albert Pranoto Director of Business Development (Singapore)
Albert’s Regulatory Hint
“With the implementation of new regulations around the world and throughout the region, many international manufacturers need short-term support from time to time to meet high-volume short-term regulatory needs.”
US: +1 512 898-9222
SG: +65 8800-3197
EMAIL: inquiry@asiaactual.com
実際の亞洲
เอเชีย แอคชวล
एशिया वास्तविक
실제 아시아
515 Congress Avenue, Suite 2100
Austin, TX 78701
+1 512 898-9222
Contact Us
Privacy Policy
116 Changi Road, #04-05
