South Korea Medical Device Market
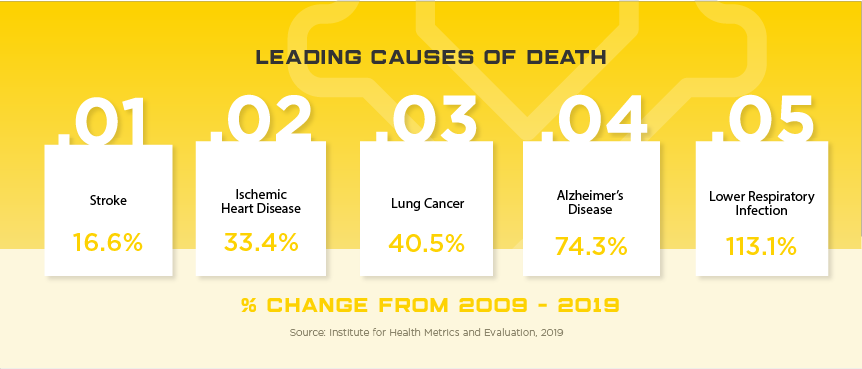
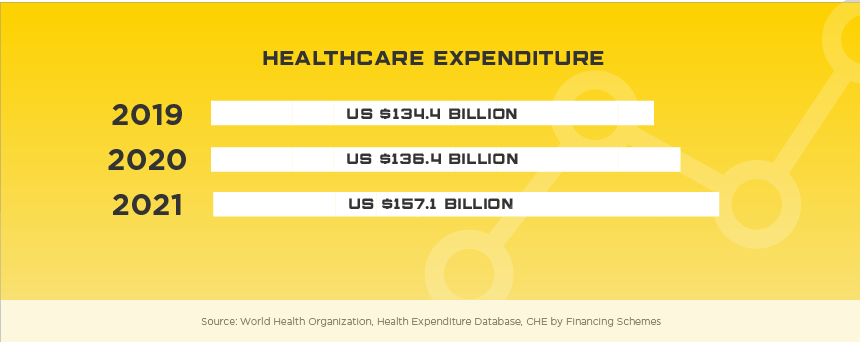
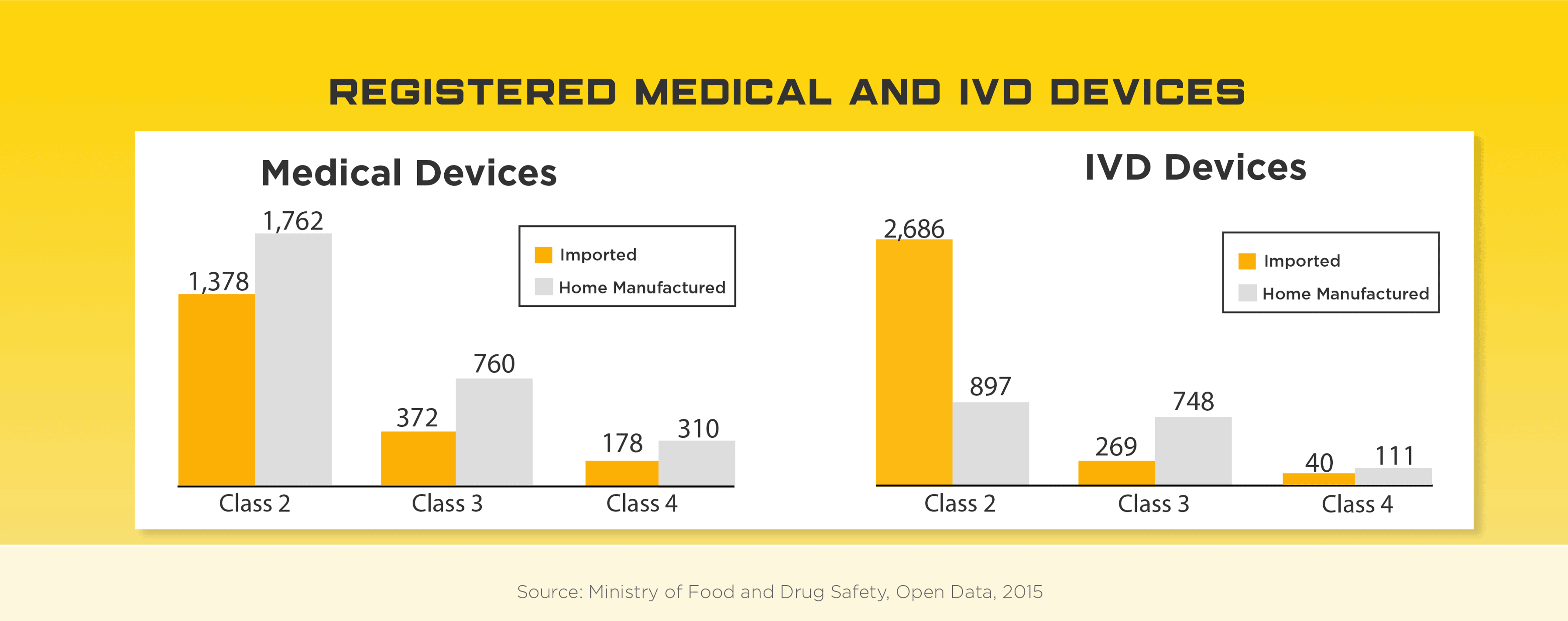
Sitting on the tip of the Korean peninsula, the South Korea medical device regulatory system is highly sophisticated and provides their citizens with high quality medical devices and adequate safety oversight. Considered a success story on multiple accounts, South Korea’s high-tech, service-based economy has led to one of the world’s highest per capita income populations and the 11th largest economy in the world. As a tech hub, the South Korean medical device market has developed a sizable local industry but still relies on foreign manufactures for over half of their medical device needs, specifically high-end. In 2019, total medical device imports were estimated at US$3.7 billion with US companies holding about 47% of this market. Some high potential areas include: knee joint prostheses, CT systems, MRI devices, intravascular catheters, dialyzers for hemodialysis, soft contact lenses.
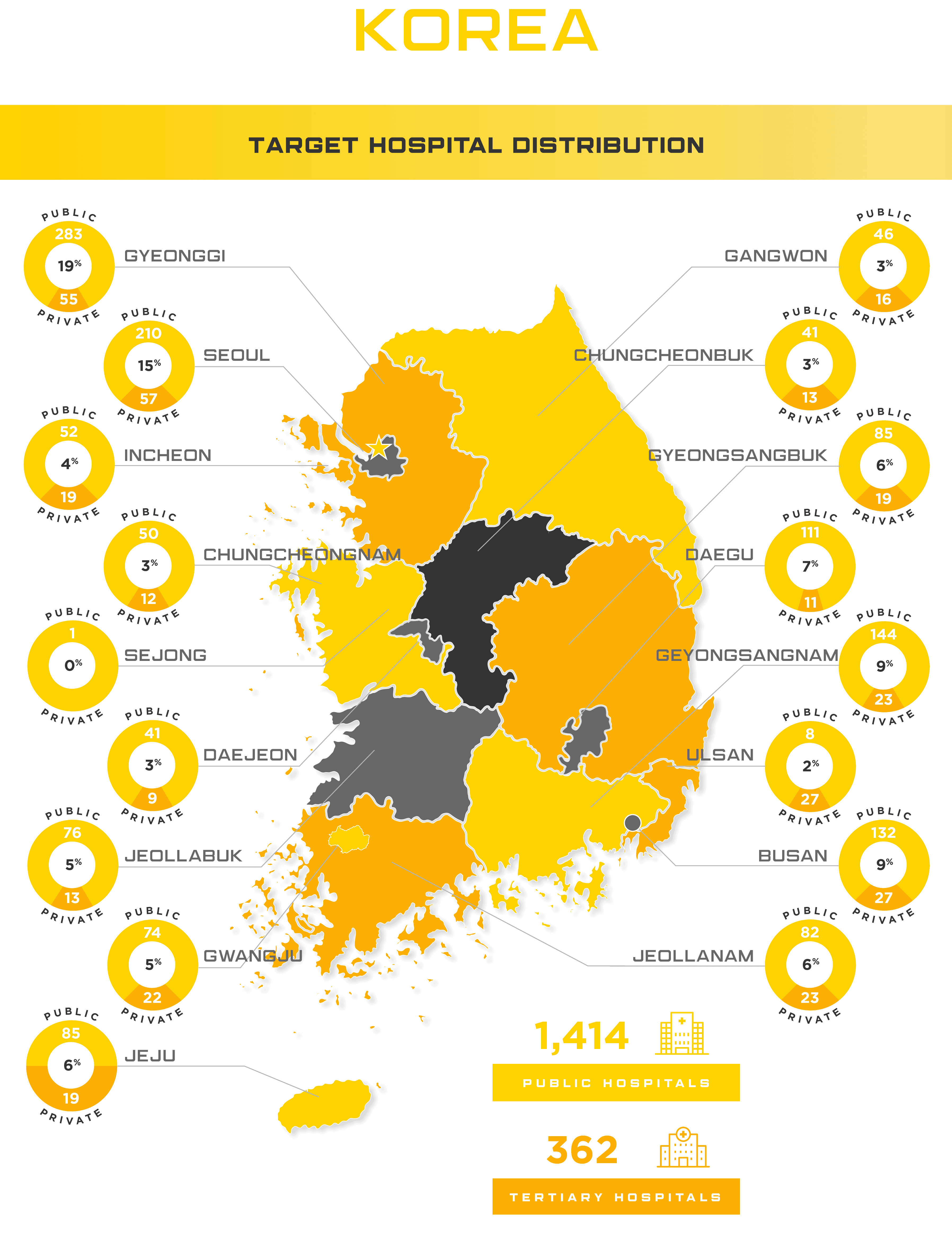
Once approved to sell your device in South Korea, it’s vital to negotiate prices with the Korean government as the National Health Insurance (NHI) system is compulsory and covers all 51 million of their citizens. Even though it’s compulsory, 97.5% of Koreans still pay a premium to have the right to use any physician or medical institution. Korea’s Ministry of Food and Drug Safety (MFDS) has also recently introduced their Unique Device Identifier (UDI) system as part of Integrated Medical Device Information System (IMDIS) in order to support pricing controls and better post-market vigilance oversight.
GROW WITH US
Asia Actual is available to help navigate the complex medical device registration requirements and regulatory pathway for medical device and IVD distribution in South Korea.
Contact Asia Actual for a free consultation discussing the potential for your medical device or IVD in the South Korean market.
Important Documents and Links
South Korea Regulatory Support
South Korea Sales Support

Sarah Baik
Contact Us
US: +1 512 898-9222
SG: +65 8800-3197
EMAIL: Korea@asiaactual.com
Latest Market Updates
 Identifying Predicate Devices in India, Taiwan, China, Japan, and KoreaOctober 25, 2022 - 12:28 pm
Identifying Predicate Devices in India, Taiwan, China, Japan, and KoreaOctober 25, 2022 - 12:28 pm How New EU MDR Requirements Will Affect Registrations in AsiaAugust 12, 2022 - 2:07 pm
How New EU MDR Requirements Will Affect Registrations in AsiaAugust 12, 2022 - 2:07 pm Medical Device Advertising Requirements in AsiaJuly 22, 2022 - 10:08 pm
Medical Device Advertising Requirements in AsiaJuly 22, 2022 - 10:08 pm Korea MFDS Implements Medical Device Monthly Reporting RequirementsJune 28, 2022 - 12:30 am
Korea MFDS Implements Medical Device Monthly Reporting RequirementsJune 28, 2022 - 12:30 am Asia Actual is the Best Medical Device Consulting Company in AsiaFebruary 24, 2022 - 3:23 pm
Asia Actual is the Best Medical Device Consulting Company in AsiaFebruary 24, 2022 - 3:23 pm
実際の亞洲
เอเชีย แอคชวล
एशिया वास्तविक
실제 아시아
Asia Actual, LLC
515 Congress Avenue, Suite 2100
Austin, TX 78701
+1 512 898-9222
Contact Us
Privacy Policy
Asia Headquarters
116 Changi Road, #04-05