Australia
Medical and Diagnostic Device Consulting
Medical and Diagnostic Device Consulting

Regulatory oversight of medical devices in Australia falls under the Therapeutic Goods Administration (TGA), with listed products accessible on the Australia Register of Therapeutic Goods (ARTG). Foreign companies typically pursue registration through an abridged pathway by demonstrating previous approvals from recognized entities:
Foreign manufacturers must collaborate with an Australian Sponsor, such as Asia Actual, to facilitate the application process effectively.
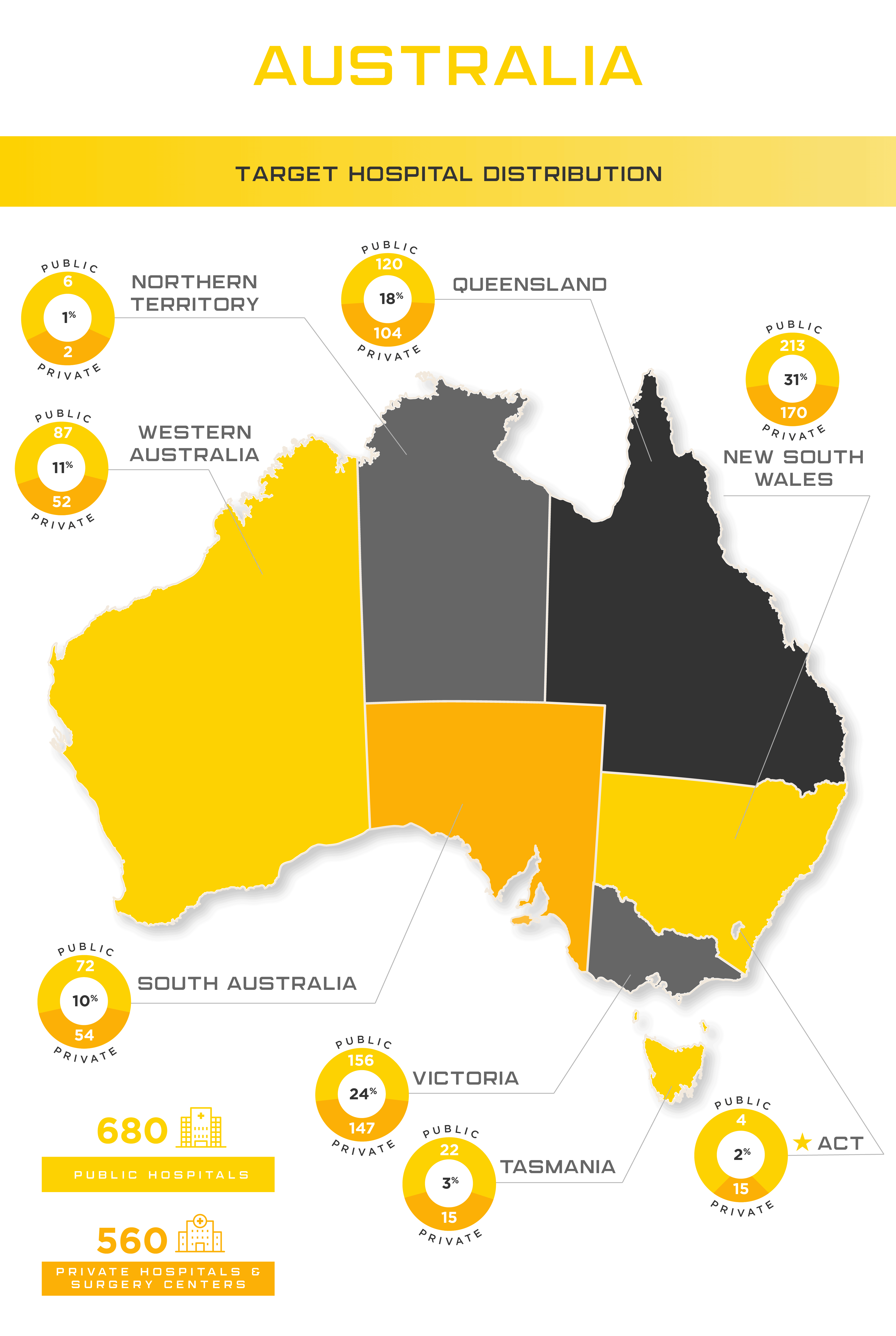
Australia’s substantial and aging population, coupled with an increasing prevalence of chronic diseases, underscores a growing demand for medical devices. Healthcare expenditure in the country has risen significantly from $180.40 billion in 2015 to $195.70 billion in 2018. Australia’s medical device industry fosters innovation, presenting favorable opportunities for manufacturers of novel devices.
Asia Actual Australia is headquartered in Sydney and managed by regulatory expert, Jack Liang. Jack and his team are available to help you navigate the medical device and IVD registration requirements and regulatory pathways in Australia.
Contact Asia Actual for a free consultation on the market access potential of your medical device or IVD in Australia.
US: +1 512 898-9222
SG: +65 8800-3197
EMAIL: Inquiry@asiaactual.com
実際の亞洲
เอเชีย แอคชวล
एशिया वास्तविक
실제 아시아
515 Congress Avenue, Suite 2100
Austin, TX 78701
+1 512 898-9222
Contact Us
Privacy Policy
116 Changi Road, #04-05
