Come grow with us in the US in Thailand in China in Korea in the Philippines in Taiwan in Hong Kong
Contact Us
US: +1 512 898-9222
SG: +65 8800-3197
EMAIL: Inquiry@asiaactual.com
VietNam Clearing Backlog of Medical Device Registration Applications
Published on: September 16th, 2024
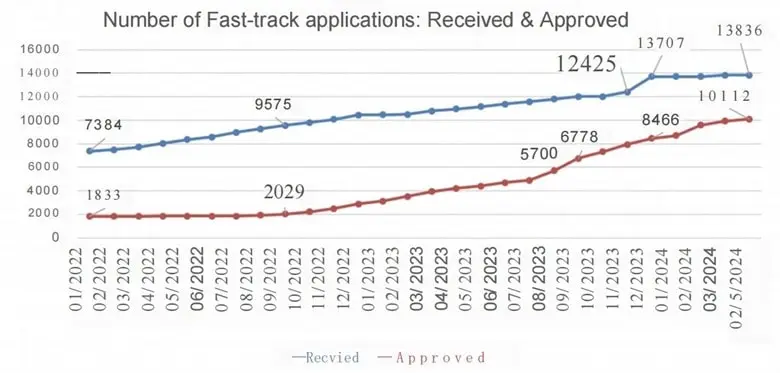
Based on our survey of the MoH database, as of May 9, 2024, the Vietnam Ministry of Health (MoH) has seen a substantial increase in the processing of Class C and D medical device applications. A total of 13,836 applications have been submitted for fast-track review between January of 2022 and May of 2024. During that period a total of 10,112 applications were closed (e.g., approved, withdrawn, or cancelled).
Class C and D Medical Device Application Processing Times
Since the introduction of the fast-track application review system in 2021, the Vietnam MoH has struggled to process applications in a timely manner. Instead of the 30 day target review times, applicant experience 10 to 16 months review times.
The chart below shows the number of fast-track applications received and approved from January 2022 to May 2024. The blue line represents the total applications received, while the red line shows the applications approved over time. Notably, there has been a steady increase in both submissions and approvals, particularly since late 2022.
From January to August 2022, only about 200 applications were processed, but the pace picked up significantly from September 2022 onwards. To date, 5,396 applications have been fully processed, including 3,268 approvals.
Total Applications Fully Processed: 5,396
Approvals: 3,268
Withdrawals: 4
Refusals: 128
Cancellations (due to missed deadlines or withdrawal requests): 2,008
Applications Appraised but Pending Approval: 4,532
-
- These applications are awaiting supplemental information, which applicants are required to provide to meet regulatory requirements.
Implications for Medical Device Manufacturers
For manufacturers, this trend processing could mean quicker access to the Vietnamese market, if applications are complete and meet the MoH’s standards.
Our expertise in navigating Vietnam’s regulatory environment ensures that your Class C and D medical device registrations are managed efficiently. If you are planning to register your medical devices in Vietnam or need support with existing applications, contact us.
Grow Medical Device Sales
with
Asia Actual
Asia Actual is a leading medical device consultancy in Asia, specializing in medical device registration and regulatory support to help manufacturers increase sales in challenging Asian markets. We offer comprehensive services, including independent license holding, direct fulfillment, and strategic sales channel support, to ensure your medical devices succeed in the region.