Come grow with us in the US in Thailand in China in Korea in the Philippines in Taiwan in Hong Kong
Contact Us
US: +1 512 898-9222
SG: +65 3138-4148
EMAIL: Inquiry@asiaactual.com
Sources and Links
Navigating the New Vietnamese Public Medical Device Pricing Portal
Published on: April 7th, 2021
The VietNamese MoH Continues to Expand Online Presence and Transparency
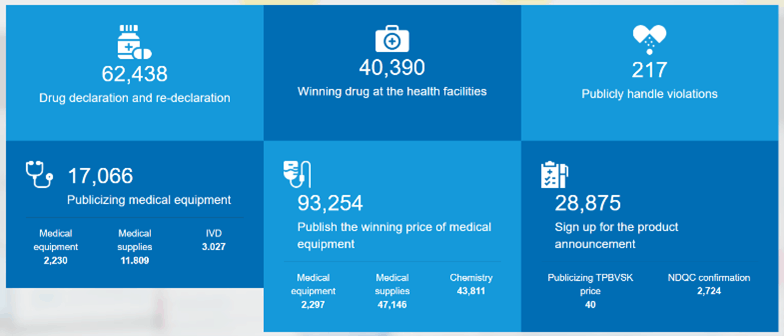
At the end of November, the VietNamese Ministry of Health (MoH) took steps to increase transparency in the medical device and pharmaceutical industries by opening the new Public Medical Device Pricing Portal. The MoH also opened the Medical Device Disclosure Portal in early September, where manufacturers can create an account and input information on their products, including prices. More information on the Disclosure portal can be found here. Once the manufacturer’s listings have been submitted through the Disclosure portal, the information is provided in a user-friendly search. Additionally, this info is also displayed in the new Public Medical Device Pricing Portal where VietNamese medical equipment buyers can review their options and contact sellers.
The New Medical Device Pricing Portal
The new Medical Device Pricing Portal was opened to the public on November 20th with the goal of setting new international standards for medical device sales transparency. The medical device section is split into 2 sections, the Medical Equipment Price and Pricing Information on Winning Bids. On one side, users can view product listings and on the other, review all final government purchases, including contracted pricing.
Medical Equipment Prices
The search is split into Medical Equipment, Medical Supplies, and IVDs. Searches here are restricted to model information, equipment group number (more information on this below), manufacturer, and list price. While information can be found here, searching by product categories is easier on the Medical Device Disclosure portal.
Winning Bids Information
The second piece of the new Purchasing portal is the Winning Bids section, where the public, local buyers, and competitors, can review final contracts. This info allows manufacturers the ability to better assess their prospects in VietNam, allows local buyers to review their pricing history, and allows competitors to review bids they lost. These factors require manufacturers to develop a sound pricing strategy to provide consistency and an optimal pricing strategy. Not only is the MoH publishing winning bids moving forward, but they are also publishing winning bids going back to 2017, allowing an unprecedented look into historical purchasing habits and price trends. While the Medical Device Disclosure portal requires manufacturers input the information, the Winning Bids section is provided by the public medical facilities.
Listed Price vs. Winning Bids
Even though manufacturers are required to list their products, this does not mean negotiations do not occur and final pricing (winning bids) will sometimes differ from the original list price. However, by providing a searchable database of all winning bids, competitors and regulators can review purchases for irregularities and/or improper business activities. Foreign manufacturers should coordinate closely with their local representatives to design and implement an optimal price strategy in VietNam as this new system is refined in the coming months and years.
Reporting Process for Winning Bids
Upon selection of a bid, government hospitals will be required to submit Contractor Selection report within 20 days and the MoH will post the report online within 10 days.
The following documents are some of what will be needed as part of the selection process:
- Contractor Selection Plan document
- Level documents
- Summary report of the current situation and plan for new equipment
- Meeting minutes of the Scientific Council when determining their choice
- Documents for determining the price of the package
New Medical Device Pricing Portal 1
Determining Which “Group” Your Product Falls Under
As part of listing product information, manufacturers will need to determine which Group each of their products fall under. The Groups are determined by where the product has been approved with the new system showing preference to products manufactured in Reference Countries and locally in VietNam. Group 1, for example, are products that are manufactured in a Reference Country or in VietNam and have a Certificate of Free Sales (CFS) from 2 Reference Countries. Products meeting Group 1 criteria can bid in all groups, while Group 5 products, for example, are only allowed to bid in Group 5 and 6 tenders. It is also important for manufacturers to make sure that if their products are included in multiple Group bids, that their pricing is consistent.
VietNam Medical Device Group Descriptions
| Group 1 | Made in a Reference Country or in VietNam with a CFS from 2 Reference Countries. |
| Group 2 | Not made in a Reference Country or VietNam but has a CFS from 2 Reference Countries. |
| Group 3 | Made in Reference Country or VietNam and has CFS from 1 Reference Country. |
| Group 4 | Not made in a Reference Country or VietNam but has a CFS from 1 Reference Countries. |
| Group 5 | Made in VietNam or those already having a local circulation number. |
| Group 6 | All other products. |
Bonus: Public Medical Facility Database
In addition to the abundance of information publicly available when it comes medical devices, the Ministry of Health also plans to maintain detailed information on medical facilities throughout the country. In the Healthcare section of the new portal, users can find a list of medical facilities by city and region, and also search by Hospital class and see specific information on each, such as departments, number of doctors, quality of service, service prices, drug prices, purchasing history, and practitioners.
Grow with Us
Asia Actual specializes in helping medical device manufacturers grow their sales in Asia with experienced, bi-lingual commercial and regulatory experts on the ground in each market. Our commercialization experts can help you evaluate the VietNamese market and develop an optimal pricing strategy that can help you grow your sales in the 100-million-person market. Contact Asia Actual today with any questions or support requests.