Come grow with us in the US in Thailand in China in Korea in the Philippines in Taiwan in Hong Kong
Contact Us
US: +1 512 898-9222
SG: +65 8800-3197
EMAIL: Inquiry@asiaactual.com
Products Unfrozen Due to COVID Needs
Due to the increasing number of COVID-19 cases in Indonesia, the MOH decided to unfreeze the following items on July 7, 2021 which had been frozen since June 18, 2021:
- HFNC (High Flow Nasal Cannulas)
- CPAPs
- Emergency Ventilators
- Continuous Ventilators (Non Invasive)
- Ventilator for ICUs (Invasive)
- Oxygen Generators
- Oxygen Generator Systems
- Portable Oxygen Generators
- Oximeters
- Patient Monitors
- Infusion Pumps
Products Currently Frozen on e-Catalogue
| Medical ultraviolet air purifier |
| Mechanical walker |
| Hyperbaric chamber |
| Hypodermic single lumen needle |
| Piston syringe |
| Dye and chemical solution stains |
| Alcohol Swab |
| Surgical apparel |
| Personnel protective shield |
| Arm sling |
| Steam sterilizer |
| Neonatal incubator |
| Neonatal transport incubator |
| Neonatal phototherapy unit |
| Blood specimen collection device |
| Blood storage refrigerator and blood storage freezer |
| Absorbent tipped applicator |
| Crutch |
| Dental chair and accessories |
| Dental operative unit and accessories |
| Liquid chemical sterilants/high level disinfectants |
| General purpose disinfectants |
| Surgical drape and drape accessories |
| Electrocardiograph |
| Radiographic film illuminator |
| Medical ultraviolet water purifier |
| Tuning fork |
| High flow humidified oxygen delivery device (Unfrozen on July 7, 2021 due to COVID needs) |
| Single/multiple component metallic bone fixation appliances and accessories |
| Smooth or threaded metallic bone fixation fastener |
| Infusion pump (Unfrozen on July 7, 2021 due to COVID needs) |
| Intravascular administration set |
| Manual surgical instrument for general use |
| Obstetric-gynecologic general manual instrument |
| Medical absorbent fiber |
| Nonresorbable gauze/sponge for external use |
| Hydrophilic wound dressing |
| Nonabsorbable gauze for internal use |
| Mechanical wheelchair |
| Infrared lamp |
| Surgical lamp |
| AC-powered medical examination light |
| Blood pressure cuff |
| Medical disposable bedding |
| Medical chair and table |
| Obstetric table and accessories |
| Manual operating table and accessories and manual operating chair and accessories |
| Operating tables and accessories and operating chairs and accessories |
| Operating tables and accessories with modification |
| Radiologic table |
| Nasal oxygen cannula (High Flow Nasal Cannula (HFNC) unfrozen on July 7, 2021 due to COVID needs) |
| Nebulizer |
| Portable oxygen generator (Unfrozen on July 7, 2021 due to COVID needs) |
| Oxygen mask |
| Cardiac monitor (including cardiotachometer and rate alarm) |
| Patient care reverse isolation chamber |
| Ophthalmic eye shield |
| Mattress cover for medical purposes |
| Infant radiant warmer |
| Medical recirculating air cleaner |
| Sterilization wrap |
| Tongue depressor |
| Body waste receptacle |
| Skin marker |
| Needle destruction device |
| Elastic bandage |
| Medical adhesive tape and adhesive bandage |
| Fetal ultrasonic monitor and accessories |
| Specimen transport and storage container |
| Oximeter (Unfrozen on July 7, 2021 due to COVID needs) |
| Sharp container |
| Patient examination glove |
| Stethoscope |
| Hand-carried stretcher |
| Wheeled stretcher |
| Powered patient transfer device |
| Manual patient transfer device |
| Powered suction pump |
| Vacuum-powered body fluid suction apparatus |
| Pediatric hospital bed |
| AC-powered adjustable hospital bed |
| Hydraulic adjustable hospital bed |
| Manual adjustable hospital bed |
| Noninvasive blood pressure measurement system |
| Human chorionic gonadotropin (HCG) test system |
| Human chorionic gonadotropin (HCG) test system (strip & midstream) |
| Clinical electronic thermometer |
| Infusion stand |
| Patient scale |
| Stand-on patient scale |
| Cane |
| Cane, crutch, and walker tips and pads |
| Anesthetic cabinet, table, or tray |
| Urine collector and accessories |
| Patient lubricant |
| Visual acuity chart |
Indonesia Issues New Guidance for Freezing Medical Devices on eCatalogue
Published on: August 5th, 2022
On July 6, 2022, the Ministry of Health released updated guidance KMK No. HK.01.07/MENKES/1258/2022, found here in Indonesian and English, outlining the process for freezing eCatalogue product categories. This comes a year after the first 79 categories were frozen and provides manufacturers with more information on the decision making process. The eCatalogue, locally referred to as the e-Katalog, can be found here.
The freezing process applies to foreign manufacturers that produce products outside of Indonesia. The intention of this new policy is to encourage the local production of certain types of medical device products and a full list of the 79 categories can be found at the bottom of the article as well as a list of items unfrozen to help fight COVID-19.
Implications of Frozen eCatalogue Product Categories
Manufacturers entering the Indonesian market will want to consider the potential implications of this new process. Specifically, manufacturers of general use or “me too” should keep an eye on the number of domestically produced similar products since their product category will likely be frozen once domestic production can meet the national demands. At this time, there is no established appeal process for manufacturers to utilize should they disagree with the MOH’s freeze status on their product category.
On the other hand, manufacturers of innovative devices or product types not currently being produced in Indonesia should feel safe that their products won’t be frozen given there is very little, to no, domestic competition and won’t be for a while.
Lastly, manufacturers considering registration in Indonesia will want to review the list of frozen product categories in order to avoid obtaining a registration for a product that can’t be sold to a significant portion of the Indonesia hospitals. Per data from the MOH, of the 2,813 hospitals in Indonesia in 2019, 63-64% are run by private organizations.
Mechanisms for Freezing Medical Devices
At this time, there are 79 categories currently frozen, including many low risk, common devices such as blood specimen collection devices, neonatal incubators, disinfectants, electrocardiographs, etc. By freezing these product categories, international manufacturers are barred from listing their products on the eCatalogue which facilitates all government purchases. Therefore, product categories added to the frozen list could significantly affect sales for international manufacturers if they solely rely on public hospital sales in Indonesia.
Once a product category is frozen, the MOH will monitor the domestic supply to ensure production can meet demand. If production is sufficient, the product category will likely remain frozen. However, if hospitals begin having issues sourcing products, the MOH will consider unfreezing product categories to allow for the importation and sale of foreign produced products.
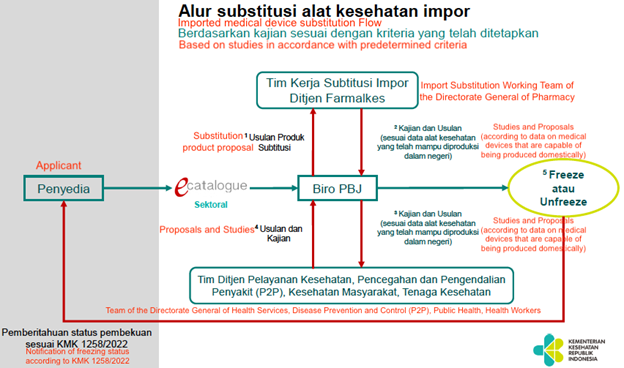
In regards to evaluating new eCatalogue listings, the Indonesian government is currently forming a substitution team to review products submitted to the eCatalogue. The team will then determine whether the product is frozen or un-frozen with a timeline of 7 working days. If the product is included in the freeze criteria, then it won’t be allowed on the eCatalogue and will be permitted the unfrozen category. The MOH has provided the below process overview to help stakeholders better understand the decision-making process.
Implementation Process
With the release of the new guidance, the MOH is quarantining new eCatalogue listings submitted after July 6th to ensure none of the products are found on the frozen list. It’s then expected that the MOH will unfreeze permitted product listings, allowing domestic public healthcare providers to purchase the products. Moving forward, the Import Substitution team will review all new listings within 7 days to ensure frozen products are not being listed and made available for purchase.
Producing Medical Devices in Indonesia
As previously noted, the goal of the new policy is boost domestic production while also encouraging international manufacturers to shift some of their production to Indonesia. When first implemented in 2021, the MOH estimated that the freezing of the initial 79 product categories would benefit the sale of 5,462 licensed medical devices and could be worth an estimated IDR 6.5 trillion (~US$100 million).
Manufacturers that wish to produce products locally and reduce the risk of having their products frozen will need to manufacture locally and obtain an AKD license designating the product as “Domestic” whereas products susceptible to the freeze will have AKL licenses. Manufacturers could also work with local companies to repackage or reassemble their products to meet these requirements.
Enrolling in the eCatalogue and Developing a Strategy
Under the new process, enrolling in the e-Catalogue will be easier than ever for manufacturers. MOH not only removed the price negotiation step but has also extended the enrollment window to almost 9 months. Manufacturers will need to work with their local partner to submit the necessary documents which includes the company deed, the medical devices license (AKL), and more.
It will also be important for manufacturers to develop and implement an e-catalogue pricing strategy. Manufacturers still have a month or two before all products have been moved over to the Sectoral catalogue but once this has been completed, manufacturers and their partners will be able to update daily if they want. Prices will be non-negotiable and products will be sold at the listed price. This varies from the similar system in Vietnam where prices can be negotiated down from the listed price but since hospitals are required to publish the final price publicly, everyone is still able to see the final price.
To learn more about the Indonesia e-Catalogue system, please see our landing page here.
To learn more about the Vietnamese Medical Device Disclosure Portal please see our blog and landing page here.
Come Grow With Us
Asia Actual works closely with clients to optimize pricing and obtain the fastest eCatalogue listing for your devices. Contact Asia Actual if you have questions regarding this new policy and/or need support with your Indonesia eCatalogue Strategy or Registration. Asia Actual has offices staffed by experienced, bilingual regulatory and commercial professionals in the major capital cities of Asia including Jakarta.