Come grow with us in the US in Thailand in China in Korea in the Philippines in Taiwan in Hong Kong
Contact Us
US: +1 512 898-9222
SG: +65 8800-3197
EMAIL: Inquiry@asiaactual.com
Thai FDA Implements Submission Process for Screening Application Documents
Published on: August 8th, 2022
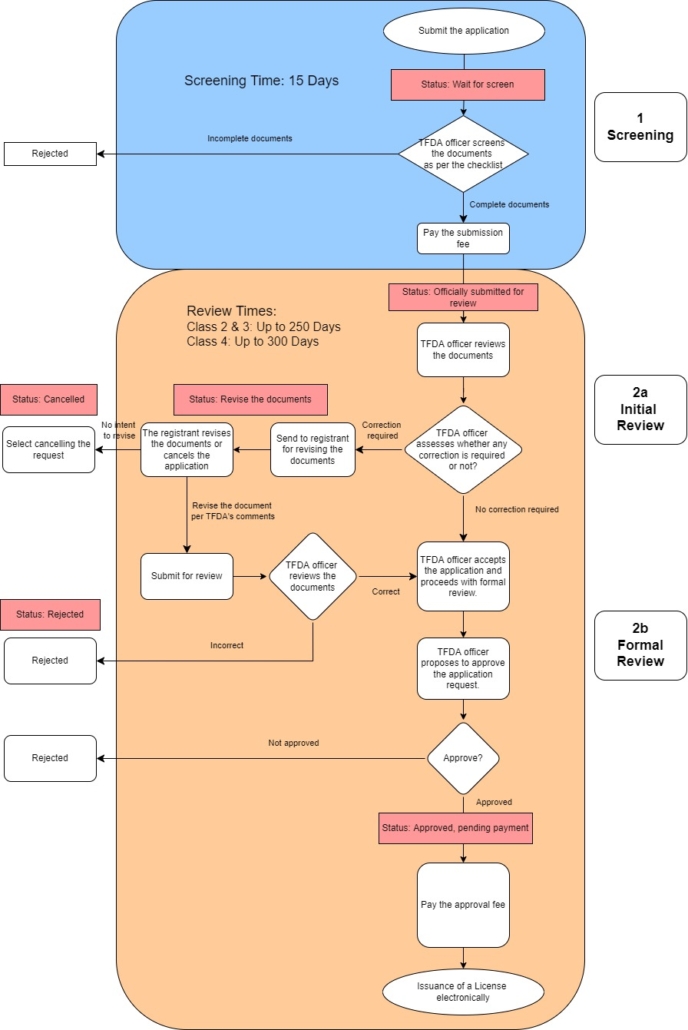
Beginning August 1st, 2022, the Thai FDA implemented a new submission process (official announcement) that will provide an initial screening of the documents provided and a response within 15 days. The new process is applicable to all applications except for Class 1, which are auto-approved, and Fast Track applications. This comes as the TFDA implements updated processes, such as query response timelines, following the recent implementation of their new medical device regulations. This latest update addresses the initial steps the TFDA will take in the first 15 days after receiving a new application which includes Screening and Reviewing the application. All Thai FDA applications must submitted through the e-Submission portal.
New Submission Process Overview
The process is similar to a pre-submission process but instead of confirming classification and/or grouping strategy, the TFDA’s Screening and Review will be focuses on the documents provided.
Step 1: Screening
The Screening step will check for the completeness of the documents according to the TFDA’s checklist (English). The documents uploaded must match the specified topics and if the documents are incomplete, the application will be rejected immediately. If the documents are complete, TFDA will allow the application to proceed to the Review step and the submission fee will be charged.
Step 2: Review
After the submission fee transaction has been completed, the application will be assigned to the Reviewer. During this step in the process, the reviewer will assess the documents for their correctness and evidence of safety and efficacy. Should the TFDA officer find incorrect or incomplete documentation, they will provide feedback and will allow the registrant to amend their documents in response to comments within 15 calendar days, 1 time. If the amendment cannot be made within the specified timeline, the application will be rejected. The 15 calendar days does not include any additional time required after the application has been submitted for approval by the authority as well as answering expert feedback should the application besubjected to an expert review.
For more information on the registration process in Thailand, please see our Thailand Medical Device Registration page.
Come Grow with Us
Asia Actual has an office in Singapore and Bangkok staffed by experienced, bilingual regulatory and commercial professionals to address any questions or provide support. Please contact us to explore if this new program is appropriate for your devices.
Asia Actual is a regulatory consulting company specializing in helping manufacturers grow their sales through independent license holding, direct fulfillment, and a variety of sales channel support services.