Come grow with us in the US in Thailand in China in Korea in the Philippines in Taiwan in Hong Kong
Contact Us
US: +1 512 898-9222
SG: +65 8800-3197
EMAIL: Inquiry@asiaactual.com
An Introduction to GeM, India’s Online Portal for All Public Hospital Purchases
Published on: May 30th, 2023
In 2016 the Indian government launched an online portal, the Government e-Marketplace (commonly referred to as GeM) under the Ministry of Commerce and Industry to provide for efficient and transparent procurement by government agencies across the country. Limited public access to the site is available here, but requires an Indian IP address.
The system is being adopted in stages by all government agencies and currently covers the sale of medical devices to all public hospitals, clinics and other government facilities in India which delivers 75% of all healthcare in India. It is estimated that virtually all hospitals are registered with purchasing accounts in GeM and 95% of all hospital purchases are transacted via the site.
Medical device manufacturers with products registered with the CDSCO for importation and sale in India should be aware of this convenient and effective way to monitor and control pricing to all Indian public institutions.
If you have questions about the GeM portal, how to access the site/list products, or about medical device registration in India more generally, feel free to contact us here.
Features and Advantages of the GeM Portal
The goal of the GeM portal is to bring transparency, efficiency, and security to the process of government procurement. It offers the opportunity for manufacturers/importers to reach government hospitals and clinics in all regions of India with consistent messaging.
With the GeM portal, the procurement process for government agencies and vendors is much more straightforward, reducing the time & resources required to purchase goods and services, as well as providing a new platform for small to medium-sized businesses to participate in government procurement.
Here are some of the features/benefits of the India GeM portal that medical device manufacturers can take advantage of:
- Provides pricing, branding, and other product information,
- Reporting/analytics features,
- Vendors can participate in bidding processes and win government contracts,
- Real time tracking of orders,
- Product pricing history.
GeM Portal Layout and Accessibility
The GeM portal currently can only be reached by an Indian IP address (the URL can be accessed here). Below are several screenshots showcasing various aspects of the site, how it works and how to best utilize it.
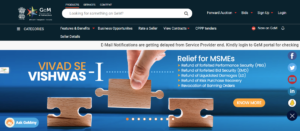
The GeM homepage as of 4/18/2023.
This page serves as the main access point for government facilities to access products, including medical devices. Access to all products can be found via this page.
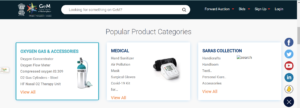
Popular Product Categories for India Government entities.
The Product Categories page features a series of different categories and sub-categories, organized with ease of use in mind. Accessible from the homepage, this allows a government organization to easily find the products they are interested in purchasing on behalf of their entity (such as medical devices/products).
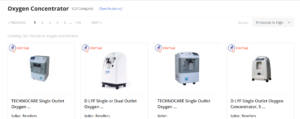
Various product listings.
After searching for a specific device, a series of product listings (in this case centralized under “Oxygen Concentrator,”) appear, allowing Government entities to easily browse through products based on their needs.
Additional Designations and Differentiators
Several other important features/requirements to keep in mind for manufacturers/distributors interested in registering their products on the GeM portal are as follows:
- Startups get their own label,
- As of 2022, 12,165 start-ups were registered on the GeM portal, representing a total of 8,200 products registered on the portal,
- Products from women led start-ups/female entrepreneurs are eligible for the “Womaniya” label within the GeM portal,
- This initiative works to encourage the participation of women entrepreneurs in the GeM portal,
- Provides Sold by Information,
- Resellers will need the authorization of the original manufacturer in order to register the product as an OEM registered trademark. When a Brand Listing application is submitted, an application ID will be received to check the status of OEM consideration,
- Provide Quantity in Stock,
- Can provide MSRP as well as Offer Price,
- Allows products to be sorted by International Standard (IS),
- Provides Seller Rating (not testimonials),
- “Assessed” label by the Rating,
- Sellers must be screened by a “Vendor Assessment” on the GeM portal for their credentials to be approved to establish OEM and showcase possession of products intended for sale,
- 29,000+ Active Bids.
How the eCatalogues of Other Countries Compare to India’s GeM Portal
The India GeM portal is one of several currently active ASEAN government purchase portals, including the Indonesia eCatalogue (or eKatalog) and the Vietnamese Medical Device Disclosure portal. These sites were all created in service of making it more accessible for manufacturers to sell products to the government in these countries, as well as to provide further pricing transparency.
Most importantly, the GeM portal can be used to generate purchase orders much like Indonesia’s eCatalogue. However, the Vietnamese Medical Device Disclosure portal is for advertising devices and listing their pricing, not for purchasing directly.
The GeM portal is most similar to Indonesia’s platform, having the most overlap in features. GeM and the Indonesia eCatalogue both function as government marketplaces for a wide variety of products that may need to be purchased by a government entity, while Vietnam portal is strictly used for medical devices. However, currently all three government portals allow for voluntary listing.
Grow With Us
Please contact Asia Actual with questions about listing products on the Government e-Marketplace in India. Asia Actual specializes in helping medical device manufacturers grow sales in Asia with experienced, bi-lingual commercial and regulatory experts on the ground in each market. Contact Asia Actual today with any questions or support requests.
Asia Actual is a regulatory consulting company focused on helping manufacturers grow sales in challenging Asia markets through product registration, independent license holding, direct fulfillment, and a variety of sales channel support services.





