“Device manufacturers should discuss with their Philippines distributors about what opportunities are available through the PCSO. This conversation should take place every year.”
Glend Llandtada
Asia Actual Philippines
Important documents and links
Philippines Releases US$9M To Hospitals For COVID-19 Response
PCSO OFFICIAL ANNOUNCEMENT
On May 22, the Philippines Charity Sweepstakes Office (PCSO) announced US$9,000,000 in funding to 87 government hospitals to support procurement of medical devices, equipment and supplies to combat COVID-19. These funds are part of a larger US$60 million package, the bulk of which goes to PhilHealth (the national healthcare system) and to cover hospital costs for COVID-19 patients. The complete list of hospitals and their funding levels is available here.
THE PHILIPPINE CHARITY SWEEPSTAKES OFFICE (PCSO)
The Philippine Charity Sweepstakes Office (PCSO) is responsible for administering Sweepstakes, national & local lotteries, scratchcards, keno and horse racing. Even though every year the PCSO is a major source of funding for new hospital construction and medical device and equipment purchases, it is often overlooked by outside suppliers.
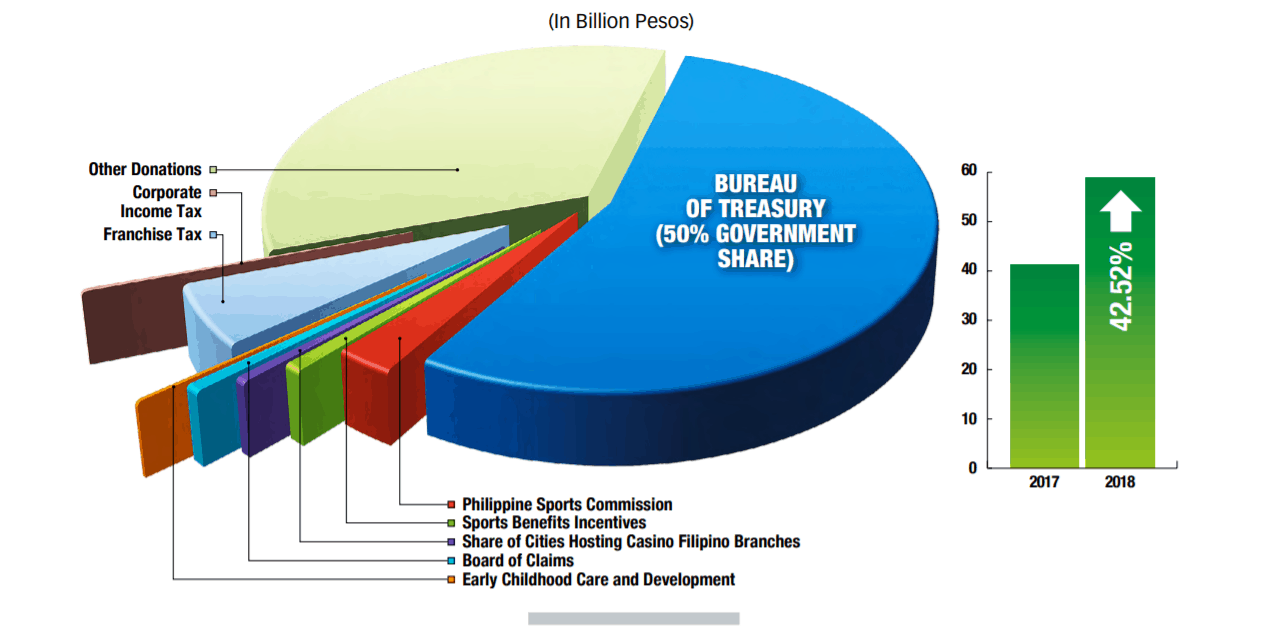
By charter every year 35% of the organization’s revenue goes to healthcare charities and other social funds which includes directly assisting Filipinos with their healthcare bills, subsidizing PhilHealth, the national insurance program, and helping hospitals purchase new equipment or supplies. For instance, in 2017, the PCSO donated PhP20M to PNP General Hospital for new medical equipment. In 2018, the PCSO posted revenues of PhP63.68B (US$125.6M), a 20% increase in revenue over the previous year. After funding the winnings and operating costs, the PCSO allocated almost $44M to healthcare causes.
Since the beginning of the COVID outbreak, the PCSO has released almost PhP3B to help fight the virus through direct payments to 82 government hospitals, dividend payments to the Department of Finance and PhilHealth (national insurance program), patient hospitalization costs, additional funds for relevant research organizations, and other local assistance like food packs, sanitizers and masks. One notable hospital that received PCSO funds, Vicente Sotto Memorial Medical Center Sub-National Laboratory (VSMMC SNL) in Cebu City, was able to use the PhP 30M to purchase additional PPE for their frontline workers while also increasing their supply of COVID testing supplies.
OVERVIEW OF PCSO FUNDING ALLOCATION
| Recipient | Amount Allocated |
|---|---|
| Department of Finance | PhP2.2 Billion |
| PhilHealth – Hospitalization costs of COVID-19 | PhP420 Million |
| Daily fund allocation to the Lung Center of the Philippines, Philippine Heart Center, Philippine General Hospital, Rizal Medical Center and Taguig Pateros District Hospital. | PhP4.2 Million |
PAGCOR HEALTHCARE FUNDING
Another source of extraordinary healthcare funding is the Philippine Amusement and Gaming Corporation (PAGCOR) which oversees casinos, e-games cafes and bingo halls. While the PCSO currently provides significantly more financial support to the healthcare industry, medical device manufacturers looking for creative ways to grow sales should keep an eye on PAGCOR as it has rapidly grown in the last 5 years with annual revenue of over PhP100B in 2018. Unlike the PCSO, PAGCOR’s primary mission is to help the Philippine government in its “nation-building programs” which allows for more discretion when it comes to allocating funds. For example, in 2018, PAGCOR helped fund drug prevention, sports development and early childhood care; with a more complete breakdown of contributions found below.
Then in 2019, PAGCOR, along with the Bloomberry Cultural Foundation, announced that they would help build and equip the new state of the art facility for overseas Filipino workers (OFWs). While originally planning to donate PhP150M (US$3M) worth of hospital equipment, PAGCOR has now increased this amount to PhP200M with Bloomberry funding the construction costs of PhP500M. The five-story, 100 bed hospital, located 65km north of Manila is expected to be finished in May of 2021 and once completed, will be equipped with dialysis and cancer centers. The Bloomberry Cultural Foundation Inc is the corporate social responsibility arm of Solaire Resort and Casino, the first complex to break ground in PAGCOR’s Entertainment City.
PAGCOR AND THEIR PARTNERS DURING COVID-19
Now with COVID stressing the healthcare sector, PAGCOR has begun providing funds and facilitating donations by the casinos and gaming operators.
Some of the notable contributions to date include:
- Okada Foundation – PhP25M (US$500k) to the Philippine Heart Center and the Lung Center of the Philippines for machines, equipment and medical supplies.
- Resorts World Philippines Cultural Heritage Foundation, Inc. (RWPCHFI) – PhP50M and 400 infrared thermometers and are expected to provide up to 144,000 additional pieces of PPE
- Melco Resorts – PhP50M (US$1M) in the form of food backs
- Bloomberry Cultural Foundation – PhP100M worth of goods, setup quarantine and treatment facilities, and donated a Polymerase chain reaction (PCR) machine (used to test for COVID-19)
OPPORTUNITIES THROUGH THE PCSO ARE AVAILABLE
Asia Actual has a strong office in Manila staffed by experienced, bi-lingual regulatory and commercial professionals to help device manufacturers stay compliant and grow sales in the Philippines. Please contact Asia Actual with questions or support requests.