Contact Us
US: +1 512 898-9222
SG: +65 3138-4148
EMAIL: Japan@asiaactual.com
Medical device registration in Japan is overseen by the Pharmaceuticals and Medical Devices Agency (PMDA) of the Ministry of Health, Labor and Welfare (MHLW) under the newly revised (last revision: 2014) Pharmaceutical and Medical Device Law (PMDL), formerly the Pharmaceutical Affairs Law (J-PAL).
The cost and time to register a medical device in Japan will vary greatly depending on device classification and, more significantly, whether the application falls under the predicate system, the Japan Medical Device Nomenclature Code (JMDN code) system.
The regulatory process in Japan can be quite complex as cost and timelines vary significantly depending on classification and the existence of predicates. Devices must be accurately evaluated and then classified as ‘new’, ‘improved’ or ‘me-too’ depending on the existence of an applicable JMDN code and registered predicates.

Low Risk Class I medical devices are subject to Pre-Market Submission (PMS) also known as ‘todokede.’ Applications are submitted to the PMDA and are considered accepted upon submission. Applications are exempted from quality systems conformity certification requirement, although manufacturer are expected to be compliant. It takes one week to issue a PMS number. There is no PMDA fee to process the application.
| Application Review | J¥ | US$ ($1= J¥148) |
|---|---|---|
| Class I | ¥0 | $0 |
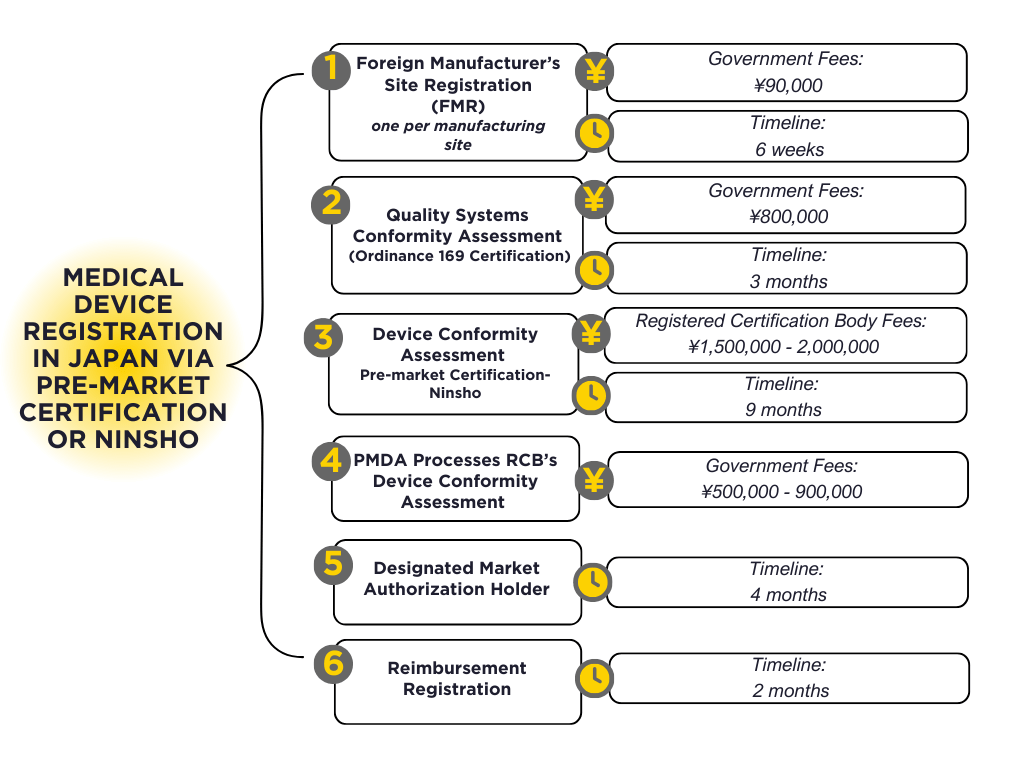
Most Medium Risk Class II and some High Risk Class III devices qualify for Pre-Market Certification (PMC), also known as ‘ninsho.’ Review of the medical device and quality systems conformity assessments are outsourced by the PMDA to Registered Certification Bodies (RCB). There are 10 RCBs, 7 of which are international companies that also offer Notified Body/Registrar services. The average time to process a PMC application is 3 months, with an average cost of US$30,000.
| Application Review | J¥ | US$ ($1= J¥148) |
|---|---|---|
| Class II: Generic (with AS)- RCB Review | ¥500,000 | $3,378 |
| Class III: Generic (with AS)- RCB Review | ¥500,000 | $3.378 |
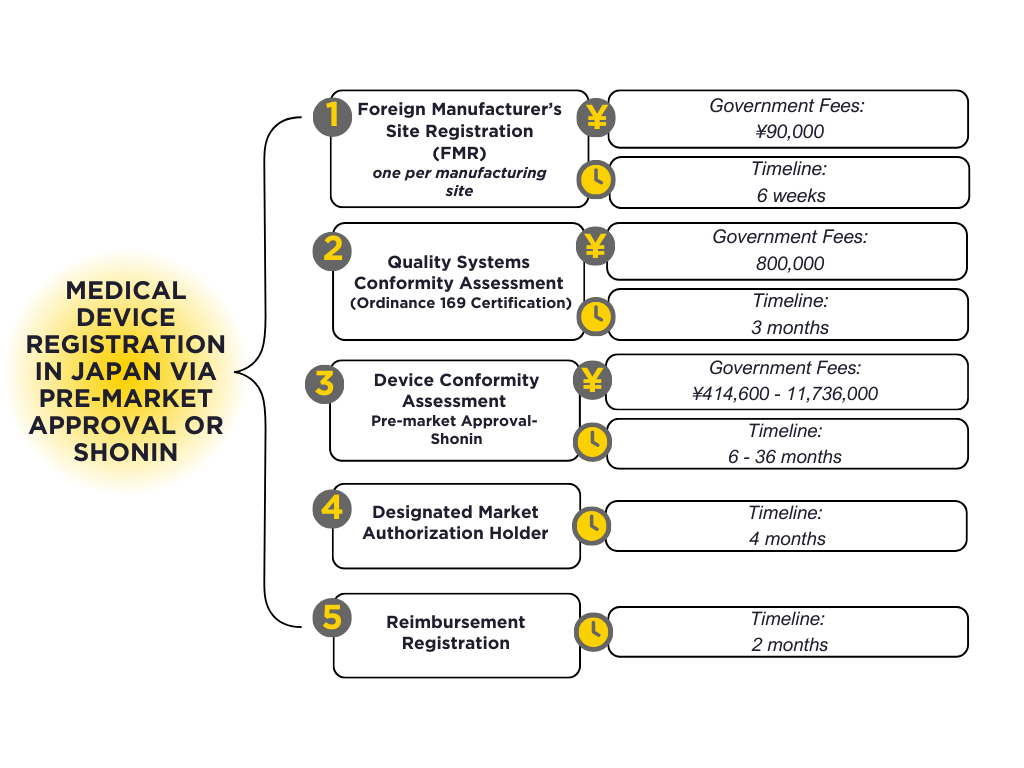
New Class II, Class III and Class IV devices are subject to Pre-Market Approval (PMA), also known as ‘shonin’ by the PMDA. Application processing time and PMDA/MHLW fees will vary from 6 months and US$20,000 to 36 months and US$120,000, depending on classification, JMDN code application, and requirements for clinical evidence.
| Application Review | J¥ | US$ ($1= J¥148) |
|---|---|---|
| Class II: Generic (without CI, with AS) | ¥414,600 | $2,801 |
| Class II: Generic (without CI or AS) | ¥1,480,400 | $10,003 |
| Class II: Improved (without CI or AS) | ¥1,480,400 | $10,003 |
| Class II: Improved (with CI) | ¥4,404,700 | $29,761 |
| Class II: New (with CI) | ¥8,620,500 | $58,247 |
| Class III: Generic (without CI, with AS) | ¥414,600 | $2,801 |
| Class III: Generic (without CI or AS) | ¥1,480,400 | $10,003 |
| Class III: Improved (without CI or AS) | ¥1,480,400 | $10,003 |
| Class III: Improved (with CI) | ¥4,404,700 | $29,761 |
| Class III: New (with CI) | ¥8,620,500 | $58,247 |
| Class IV: Generic (without CI, with AS) | ¥499,700 | $3,376 |
| Class IV: Generic (without CI or AS) | ¥1,838,200 | $12,420 |
| Class IV: Improved (without CI or AS) | ¥2,425,900 | $16,391 |
| Class IV: Improved (with CI) | ¥6,896,500 | $46,598 |
| Class IV: New (with CI) | ¥11,736,000 | $79,297 |
If clinical evidence is required as part of the registration application, it is recommended to request a formal meeting with the PMDA to determine if your data is sufficient for a successful application. The PMDA fee for the 2- hour meeting is approximately US$10,000.
Similarly, it is recommended to request a formal meeting with the PMDA to confirm if your clinical study is designed such that the data will be acceptable for a medical device registration application. The PMDA fee for the 2-hour meeting is approximately US$10,000.
Contact Asia Actual to see if we can help navigate the registration process for your medical device in Japan.
US: +1 512 898-9222
SG: +65 3138-4148
EMAIL: Japan@asiaactual.com
Who can submit submit applications in Japan?
A local entity, called a Designated Market Authorization Holder or DMAH, must submit applications, hold the license, and correspond with the Pharmaceuticals and Medical Devices Agency (PMDA).
How are products classified in Japan?
Medical devices are classified into 4 categories in Japan from low-risk Class I products to high-risk Class IV in accordance with the Japan Medical Device Nomenclature Code (JMDN code) system.
How long does it take to register my medical device in Japan?
Approval timelines will widely vary depending on the product’s risk and novelty. Low risk products with predicates already on the market will be approved quickly while new products will often require local clinical evidence which can extend review times up to 3 years. New Class II, Class III and Class IV devices are subject to Pre-Market Approval (PMA), also known as ‘shonin’ by the PMDA. Application processing time and PMDA/MHLW fees will vary from 6 months and US$20,000 to 36 months and US$120,000, depending on classification, JMDN code application, and requirements for clinical evidence.
How much does it cost to register my device in Japan?
The cost to enter the Japanese market will vary depending on the product’s classification and whether there are predicates already on the market. Class I applications do not incur a fee and will be approved upon submission while new Class II, III, or IV applications could require anywhere from US$20,000 to US$120,000 depending on the amount of clinical evidence that will be required.
However, most Class II, III, and IV products with similar products on the market will be able to work with a Registered Certification Bodies (RCB) and can get approved for about US$30,000.
How long are medical device licenses valid in Japan?
Medical device licenses do not expire in Japan.
Does the license holder need to be a part of the importing process?
No, license holders can authorize multiple importers to import under one license.
Is ISO 13485 required for medical devices in Japan?
No, ISO 13485 isn’t required but a local similar GDP requirement, will be required as stipulated in MHLW Ministerial Ordinance No. 169 (2004).
Is Home Country approval required?
No, there are no requirements for home country approval.
実際の亞洲
เอเชีย แอคชวล
एशिया वास्तविक
실제 아시아
515 Congress Avenue, Suite 2100
Austin, TX 78701
+1 512 898-9222
Contact Us
Privacy Policy
116 Changi Road, #04-05
