Medical Device Consultations in Japan
Published on: April 30th, 2024
Medical device consultation meetings with the PMDA are a very involved, but essential, process in registering medical devices in Japan. Prior to submitting a pre-market approval “Shonin” application, a significant part of the regulatory process in Japan is consultation meetings with the Pharmaceuticals and Medical Devices Agency (PMDA). In fact, most medical device applicants in Japan will need several meetings with the PMDA with each meeting serving a distinct purpose in the approval process. Applications may be submitted without consultations, but for increased speed and a higher success rate, consultations are highly recommended. Usually, the first step in gaining regulatory approval for a novel product is to seek input from the PMDA as to the regulatory pathway, classification, and data requirements for said product.
For a general overview of the Review Process by the PMDA, visit this link.
Comparing Medical Device Consultations:
PMDA to the FDA
The PMDA Consultation process serves a similar function to the Center for Biologics Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER) formal meeting process and the pre-submission process with Center for Devices and Radiological Health (CDRH) at the FDA. However, when compared to the FDA, PMDA consultations are generally deemed to be much more of a requirement, especially for novel products. The consultation process can also be completely or partially bypassed if a product aligns closely with a predicate or has prior experience with the PMDA. Unlike the FDA, PMDA consultations do require fees for most meetings.
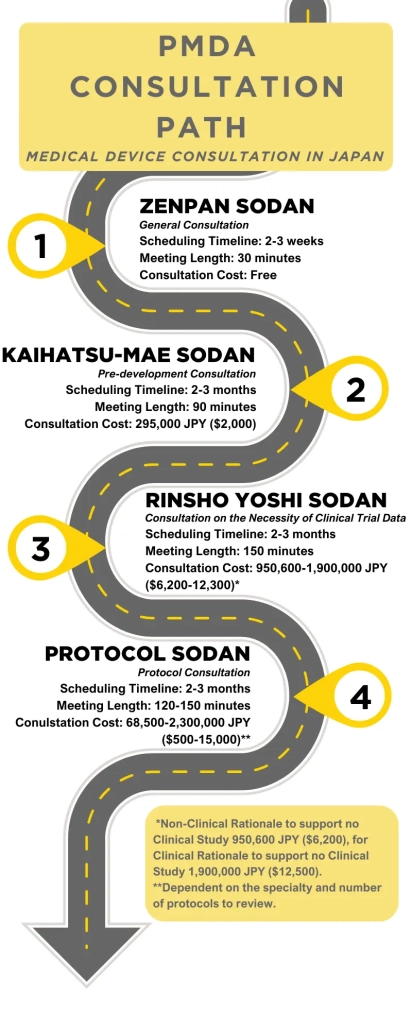
PMDA Consultation Process
The consultation process begins with a free general consultation called Zenpan Sodan and is meant to give insight to future consultations as well as to the device’s regulatory pathway. Formal consultations (other than Zenpan Sodan) consist of two meetings with the PMDA: A 30 minute long preparatory meeting called Junbi Mendan and a 1-2 hour main meeting called Hon Sodan. The Junbi Mendan is supposed to enhance the productivity of the main meeting which is why specific talking points are determined and readiness of consultation materials affirmed during the meeting. Hon Sodan meeting content will vary depending on the type of consultation at hand but usually is dedicated to delving into details from which specific feedback is derived.
Please note that these meetings are in chronological order, but may not be necessary to attend depending on feedback from the PMDA.
General Consultation: Zenpan Sodan
Initially, for sponsors seeking the PMDA’s guidance for their product development plans, a Zenpan Sodan meeting is step one. In the meeting, sponsors are expected to introduce their product and follow-up with a discussion of the regulatory pathway best suited to it. Zenpan Sodan is essential to developing a regulatory strategy and aligning regulatory expectations. Subsequent Zenpan Sodan meetings can take place throughout the consultation process to reinforce acceptability of strategy as well as to introduce high-level topics that would benefit from more general feedback.
Pre-development Consultation: Kaihatsu-mae Sodan
After the Zenpan Sodan, the PMDA usually sends sponsors to a Kaihatsu-mae Sodan. In this meeting, sponsors should provide their Non-Clinical Testing suite to PMDA for review and feedback. The manufacturer is expected to present, discuss, and ask questions related to the medical device’s specifications, materials, predicate devices, and the sufficiency of the bench testing data to support the regulatory submission. Afterwards, the PMDA will give guidance and advice on the applicability and interpretation of requirements in order to obtain approval.
Consultation on the Necessity of Clinical Trial Data: Rinsho Yohi Sodan
The PMDA uses this meeting to determine if the product has enough clinical data available to warrant marketing approval. In cases where more clinical data needs to be generated, a domestic clinical study may be required. In either case, the purpose of the meeting is to present the PMDA with the available clinical and non-clinical study data, peer-reviewed literature, and available global sales data, in order to determine if a domestic clinical study is necessary for the regulatory submission.
Protocol Consultation: Clinical Trial Protocol Sodan
Before initiating clinical trials, sponsors must seek the PMDA’s input on the conduct and design of the trial in this meeting. In the Protocol Sodan, protocol details are outlined and questioned to support a more acceptable regulatory filing. In presenting their proposed study protocols, sponsors should include their objectives, endpoints, patient population, and statistical analysis plans, to the PMDA for review and feedback. Ultimately, there are multiple disciplines for which the protocol can be discussed in this meeting: (i) Safety, (ii) Quality, (iii) Performance, (iv) Exploratory Clinical Trial, and (v) Clinical Trial. This consultation reinforces that the study is conducted in a manner that generates robust and reliable data and aligns with regulatory expectations to support its final product approval.
Making the Most of Your Consultation Process
To sum it up, if leveraged carefully, these consultations with the PMDA will enhance sponsor’s regulatory submission efficiency and increase their chances of product approval. It is highly recommended that the sponsor uses the opportunity provided by these consultations to interact with the regulatory authority, seek guidance, and ensure compliance. In no uncertain terms, engaging in PMDA meeting consultations is a fundamental aspect of the regulatory journey for pharmaceuticals and medical devices in Japan.
Come Grow With Us
Please contact us if you’d like support understanding these new requirements or are interested in registering your product in Japan. Asia Actual specializes in helping medical device manufacturers grow their sales in Asia with experienced, bi-lingual commercial and regulatory experts on the ground in each market. Contact Asia Actual today with any questions or support requests.
Asia Actual is a regulatory consulting company specializing in helping manufacturers grow their sales through independent license holding, direct fulfillment, and a variety of sales channel support services.
Blog Posts
Click here to add your own text