Australia’s SaMD Regulatory Transition Deadline
Published on: April 16th, 2024
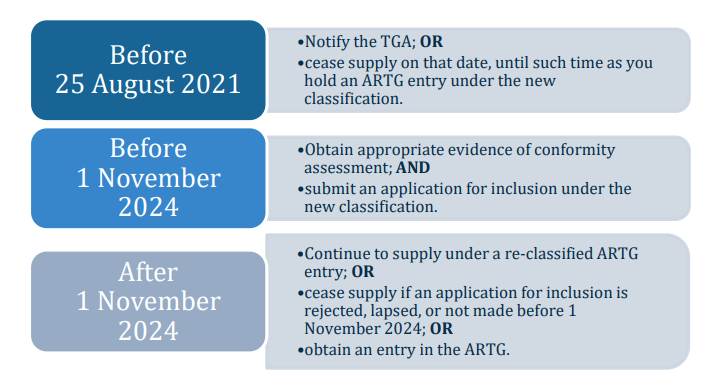
On November 1st, 2024, Australia’s Therapeutic Goods Administration’s (TGA) grace period will end and new rules surrounding software as a medical device (SaMD) will be implemented. Regulatory changes for SaMDs in Australia are a response to increasingly innovative software functions in medicine. In response to these changes, the Australian Government, alongside global medical device regulators, amended the Therapeutic Goods (Medical Devices) Regulations 2002 on February 25th, 2021.
Regulatory SaMD Changes
The changes amounted by the TGA on February 25th, 2021 will take full effect on November 1st, 2024 by which time stakeholders must have applied for a conformity assessment with the TGA. Devices passing the conformity assessment may then be included under a new, higher classification on the Australian Register of Therapeutic Goods (ARTG) list. The changes are meant to:
- Clarify regulated software product boundaries (which includes exemptions and exceptions),
- Introduce new classification rules,
- Update the essential principles so that requirements for SaMDs are more clearly expressed.
Is Your Software a Medical Device in Australia?
In order for changes to apply, the TGA must determine that the software need be registered as a medical device. Per section 41BD of the Therapeutic Goods Act (1989), a Medical Device is defined as something that meets any of the following criteria:
• Diagnosis, monitoring, prediction, prognosis, treatment or alleviation of disease, injury or disability
• Prevention of disease
• Compensation for an injury or disability
• Investigation, replacement or modification of the anatomy or of a physiological or pathological process or state
• Control or support of conception
• Is an accessory to a medical device (something specifically intended to be used together with a medical device to enable that device to function as intended)
Note these changes apply to medical device software only and are not relevant to IVD medical device software. For guidance on IVD software, see Software as in vitro diagnostic medical devices (IVDs).
Software Exclusions
As a means to prevent unnecessary regulatory oversight in Australia, certain SaMDs were carved out as being either exempted or excluded from the scope of TGA regulation.
Exclusion: Means that the devices are completely unregulated by the TGA. Excluded products typically meet the following criteria:
- Does not take data directly from a medical device.
- Is intended only for the purpose of providing a recommendation to a health professional.
- Is not intended to replace the clinical judgement of a health professional, as it is based on information (the referenced clinical guideline) that can otherwise be verified.
Excluded Clinical Decision Support Software (CDSS) is a low-risk product that might be defined as a medical device, but are not legally declared as such.
An example of excluded CDSS according to the TGA includes software modules that provides alerts for adverse drug interactions based on established rules and guidelines. (pg.8)
The types of products excluded by the TGA can often be categorized as: Consumer health products, enabling technology, digitization, population based analytics, and laboratory information management systems. Examples are digital mental health tools, software for consumers to self-manage a disease that is not considered serious, software for a patient survey, software for hospital bed management, etc. Please see a more comprehensive list here.
Software Exemptions
Exemption: Means that the TGA retains some oversight for advertising, adverse events and notification but registration of the devices is not required.
Some CDSS have been exempted, but exempt software may still be subject to some regulatory requirements.
An example of exempt CDSS according to the TGA: Software intended to compare a particular patient’s symptoms and test results with available clinical practice guidelines to recommend, to a health practitioner, condition-specific diagnostic tests, investigations, or therapy. The practice guidelines are described as the basis for the recommendation and are provided for the health practitioner’s review. (pg. 11)
For more formal guidance on Clinical Decision Support Software in Australia, follow this link.
New Classification Rules
The new classification rules will largely apply to SaMDs whose function or purpose is in: diagnosis or screening, monitoring, specification or recommendation of a treatment or intervention, and/or information as therapy. Devices in these domains may be newly classified under the new rules. Medical devices are classified according to the medical device classification rules in Schedule 2 of the Therapeutic Goods (medical Devices) Regulations 2002.
Those supplying eligible medical devices would have already had to notify the TGA of their eligibility for new classification on or before August 25th, 2021. By the end of the transition period on November 1st, 2024, germane medical device suppliers must have submitted an application for the inclusion.
Changes to Essential Principles
The TGA has established Essential Principles that all medical device manufacturers or sponsors in Australia must meet. Keep in mind that following the regulatory changes on February 25th, 2021, 2 essential principles were amended and another added.
- Essential Principle 12.1 was amended to clarify already exisiting requirements for cyber security, data and information management, and requirements related to development, production, and maintenance.
- Essential Principle 13.2(3) was amended to enable information to be provided electronically as opposed to in a leaflet or brochure.
- Essential Principle 13B was added and mandates the version and build number of software devices be accessible and easily identified by users of SaMDs. User information must also be in English.
Should you have additional questions or need help during this transition, please contact us.
Come Grow With Us
Please contact us if you’d like support understanding these new requirements or are interested in registering your product in Hong Kong. Asia Actual specializes in helping medical device manufacturers grow their sales in Asia with experienced, bi-lingual commercial and regulatory experts on the ground in each market. Contact Asia Actual today with any questions or support requests.
Asia Actual is a regulatory consulting company specializing in helping manufacturers grow their sales through independent license holding, direct fulfillment, and a variety of sales channel support services.