IMPORTING MEDICAL DEVICES INTO THAILAND
Tips for navigating a complex market
Published August 17, 2020
CUSTOMS CLEARANCE IN THAILAND
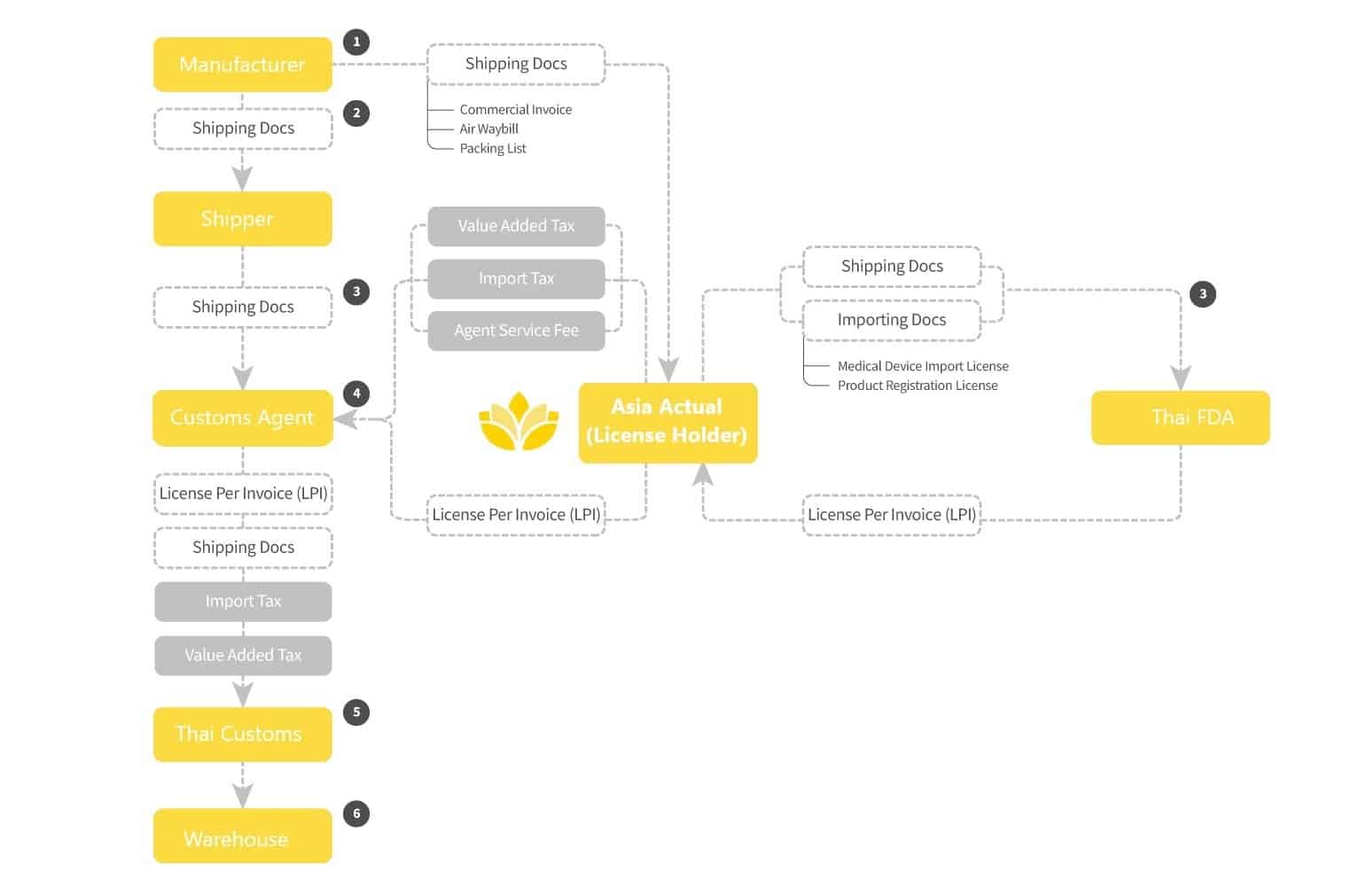
Importing medical devices into Thailand is more difficult than in other countries in the region. It is important to work with an importer with staff experienced with medical device imports to avoid customs clearance delays and added costs.
Unique to the customs clearance process for medical devices in Thailand is the requirement for a License Per Invoice (LPI) approved by the Thai FDA for each shipment. The Import License holder must apply with credentials, the original Import License, and original shipping documents (e.g., Air Waybill, shipping invoice and packing list) submitted online to Thai FDA. (The one-time fee to the TFDA for LPI submission which includes the Stamp Duty, Power of Attorney, and document submission). The FDA will confirm that all devices included in the shipment (by part number) are covered by a valid import license and then issue the LPI. The application review process take 1 to 2 days. The resulting License Per Invoice document is a required element of the customs clearance process for registered medical devices, accessories and spare parts.
THAILAND IMPORTATION DOCUMENTS & FEES
The overall process is then similar as in other countries with assemble a submission of shipping documents, payment of import tax (if any depending on HS code), payment of VAT (7% on all goods and services), etc. Shipping rate will vary significantly by mode and point of origin; and should be known to manufacturers. Customs Clearance charges should not exceed 1% of Cost of Goods.
OVERVIEW OF THE THAILAND IMPORTATION PROCESS
COMMON DELAYS FOR FOREIGN IMPORTERS
- Discrepancies or inconsistency with HS Code (when using different freight forwarders)
- Inconsistency between shipments with the value of goods
- Delayed LPI application filing
- Shipments containing expired and valid items as Customs does not allow for partial clearance
- Shipments containing parts from different manufacturers
CONTACT ASIA ACTUAL TODAY
Using Asia Actual as your License Holder in Thailand comes with the added benefit of expertly managed, efficient and transparent importations.
Contact Asia Actual for more information on our importing services.






