Important documents and links
Thailand Medical Device Market Data

BINH THAI
THAI FDA ALLOWS SALES OF AT-HOME HIV TEST KITS
On April 9, 2019, the Thai FDA released the Announcement of the Ministry of Public Health The test kit related to self-screening for HIV infection B.E. 2560, declaring that at-home HIV self-test kits can now be sold in Thailand upon regulatory approval with immediate effect.
HIV Self-Test kits still require a formal review by the Medical Device Control Division of the Thai Food and Drug Administration (FDA) according to the Medical Device Act, 2008, and Medical Device Ordinance, 2018 (as amended) as a Class 4, Licensed Medical Device a 9 to 14 month process.
Thailand Regulatory Process
Proof of regulatory approval in the country of origin had been a firm requirement for medical device registration in Thailand. However, from September of 2019 a new Concise Evaluation process was launched providing exceptions for the home-country-approval requirement (with exceptions).
Certain administrative documents such as the Free Sale Certificate and ISO certificate must be authenticated by the territorial Thai Consulate. Technical documents must be in Common Submission Dossier Template (CSDT) format as per the ASEAN Medical Devices Directive. Furthermore, Licensed medical devices are required to have performance tests generated from a local, licensed test laboratory.
Furthermore, given that the product will be used by a lay person, the Thai FDA also included specific labeling instructions such as official IFU translations and warning labels written in clear red lettering stating, “Do not use a self-screening test on people receiving antiretroviral therapy for HIV as this may result in a false negative” and “This is only to be used for manual screening and if HIV is detected, user must seek confirmation of the diagnosis from a healthcare professional.”
Thailand Registration Fees for a Class 4, Licensed device
The Thai FDA fees total ThB 98,100 (roughly US$3,110):
| 1 | Application Fee | ThB 100 |
| 2 | Expert Review Fee | ThB 88,000 |
| 3 | Approval Fee | ThB 10,000 |
Import Licenses are valid through the validity period of the Certificate of Free Sales, up to a maximum of 5 years.
Asia Actual supports medical and IVD device manufacturers grow their business throughout Asia with registration, representation and commercialization consulting services. Please contact us if you would like to learn more about how we can support your business in Asian markets.
Thai Government Focuses on Fighting HIV
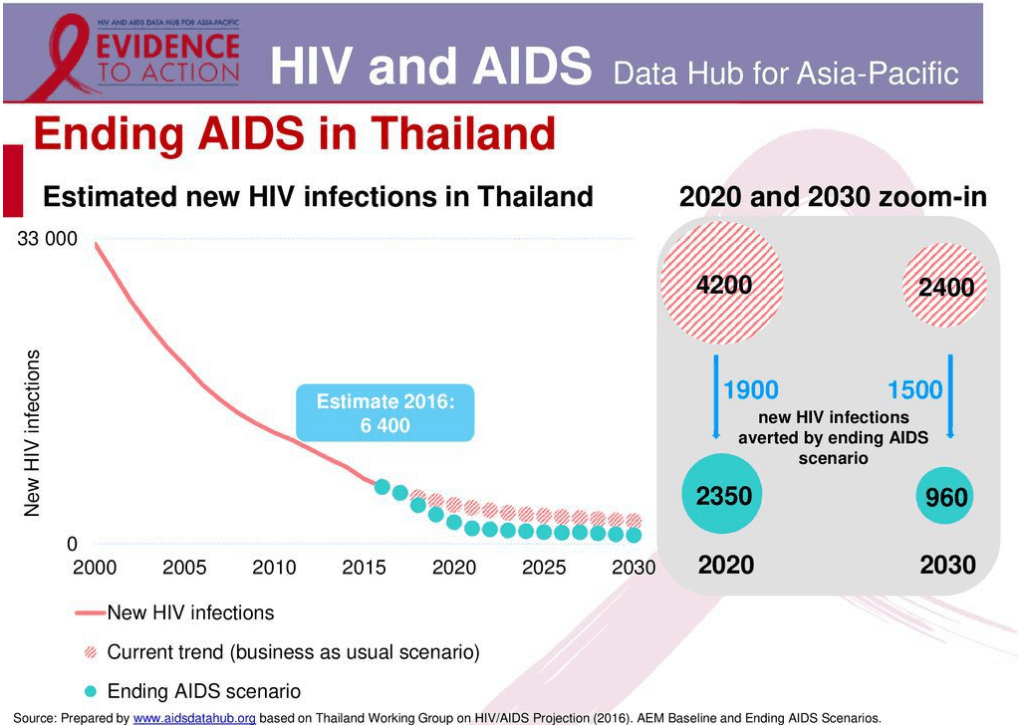
The announcement follows a global trend, partly led by the World Health Organization and locally, by Thailand’s End AIDS program, meant to help reach more people and make it easier for their citizens to monitor their health. Launched in late 2017, Thailand’s End AIDS program hopes to reduce new HIV infections from 6,500 per year to less than 1,000, cut AIDs related deaths from 13,000 to under 4,000 per year and reduce HIV-related discrimination in health-care settings by 90% by 2030.
While HIV test kits have long been available in Thailand through doctors and healthcare professionals, cheap at-home test kits are seen as a crucial next step in the fight against AIDs. Studies have shown that many at-risk demographics, such as young people, drug users or men who have sex with men, may not feel comfortable getting tested at clinics or hospitals due to the stigma associated with HIV. Therefore, by allowing people to perform these tests at home, in private, proponents hope that more people will learn their status sooner, helping to curb the spread of the disease.
As the deputy director of the SWING Foundation, Chamrong Phaengnongyang, put it, “If we want to end AIDs before 2030, we can’t wait for people to go to clinics. We’re very excited about this approval. It gives people more options as it’s faster and more convenient.” While this is seen as a big step forward by many, there are still some concerns facing Thailand and other countries permitting these at-home kits. By giving people this kind of privacy, the test kits are also removing healthcare professionals from the process which could leave vulnerable individuals without proper psychological support if they receive bad news. Though this does cause some concern, proponents argue that reducing the stigma around HIV will help with this and still doesn’t outweigh the benefit of more people knowing their status.
The Thai government has voiced their commitment to ending the epidemic by supporting preventative awareness, access to cost-effective tests and treatment resources through public investment and/or private partnerships. The Deputy Prime Minister and Chair Of The National Aids Committee, Narong Pipatanasi, stated, “The government commits to ensuring enough budget for an effective HIV response. I also would like to emphasize that strong partnerships between the government, civil society, the private sector and development partners will ensure that Thailand becomes the first country in Asia to end the AIDS epidemic.”

“The government commits to ensuring enough budget for an effective HIV response. I also would like to emphasize that strong partnerships between the government, civil society, the private sector and development partners will ensure that Thailand becomes the first country in Asia to end the AIDS epidemic.”
Narong Pipatanasai, Deputy Prime Minister and Chair of the National Aids Committee
Thai FDA History
Prior to 1988, there was no law related to control over the production, import, export, sale or handling of medical devices. The Ministry of Public Health normally solved problems related to medical devices by referring to the provisions of the Drug Acts. Due to the difference in terminology, the Medical Device Act, B.E. 2531 (1988) was enacted and had been in force since May 23, 1988. With the emergence of extensive research and development, rapidly advancing medical technology, the Medical Device Act, B.E. 2551 (2008) was promulgated to supersede the 1988 Medical Device Act.
According to the Medical Device Act, the tasks of medical device control division are to ensure effective control of safety and performance of medical devices. Activities Under the Act are supervised by the medical device committee, who give consent and advice and make recommendations to the Secretary General of the FDA. The committee can also appoint subcommittee to assist them with certain tasks. Recently, ten subcommittees have been appointed.