Come grow with us in the US in Thailand in China in Korea in the Philippines in Taiwan in Hong Kong
Contact Us
US: +1 512 898-9222
SG: +65 3138-4148
EMAIL: Inquiry@asiaactual.com
Sign Up For Our Newsletter to Stay Informed
INDIA COMPLIANCE MAINTENANCE: NOTIFIED BODY CHANGE
Published July 29, 2019
Given recent conditions of Brexit, many companies are impacted with changes regarding their notified body. This change ultimately changes the CE Mark on labeling, thus requiring amendments to any existing Import Licenses in India.
In order to remain compliant, our experts suggest to follow the post approval changes as per the Medical Device Rules 2017 (page 59)
Per the post approval change Rules, there is no specific guidance on “a change in number on the CE Mark/ Change in Notified Body”. For instance, even though the change of Notified Body from BSI UK to BSI Netherlands may not affect quality in respect of its specifications, indication for use; performance and stability of the medical device, it may still be considered a major change as there will be changes to labels (refer point below). It may also be that the approval itself is based on the CE mark (EU nation FSC submitted at the time of registration in India) and in such a case the regulator may deem this to be a major change.
Further, if we see options under post-approval changes in the online system, then for Import Licenses in Form MD-14, following options appear:
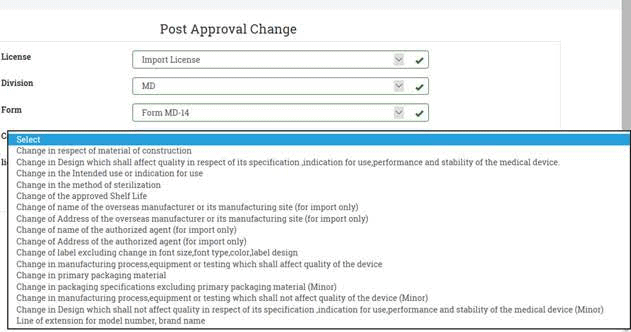
Therefore, the suggestion is to select Category 7: change label excluding change in font size, font type, color, label design
Action For Major Changes
The authorized agent, shall obtain prior approval from the Central Licensing Authority before any major change, as specified in the Sixth Schedule, is carried out and the Central Licensing Authority shall indicate its approval or rejection within sixty days.
If no communication of approval or rejection as referred to in clause above is received within the stipulated time from the Central Licensing Authority, such change shall be deemed to have been approved.
Documentation Required Would Include:
- Notarized letter from Manufacturer on their letterhead, addressed to the CDSCO explaining the details of the change and circumstances leading to the change. Letter must confirm that the change is restricted to a change of address and further confirm that there are no changes whatsoever to previously submitted site master file and device master file; no changes in manufacturing site, device registered in India, device intended use, materials of construction, manufacturing and testing processes, performance, safety, risk class, stability, biocompatibility, shelf life etc.
- Notarized copies of new certifications issued by The Notified Body, notarized CE Certificate, ISO 13485 Certificate, CE Full Quality Assurance Certificate, CE Design Examination Certificate, as applicable.
- Confirmation that this change does not require an approval in the country of origin. If it does need approval in the country of origin, then notarized evidence of such approval is to be submitted.
Contact Asia Actual
Asia Actual is available to answer questions and help navigate you through any compliance maintenance issues you may face. Contact us to arrange an initial conversation.