Come grow with us in the US in Thailand in China in Korea in the Philippines in Taiwan in Hong Kong
Contact Us
US: +1 512 898-9222
SG: +65 3138-4148
EMAIL: Inquiry@asiaactual.com
Asia Actual Launches Online Medical Device Approval Search Service
Published on: September 16th, 2021
Introducing R.O.S.E.
Asia Actual is excited to launch R.O.S.E., the Registration Optimized Search Engine. This new tool can help medical device regulatory professionals:
- Research device classification and grouping
- Identify registered predicate devices
- Monitor competitive activity
- Set license expiry alerts
The goal of R.O.S.E. is to provide centralized access to actual medical device registration data in Asian markets. Every day at Asia Actual we discuss medical device classification, grouping, and predicate identification with prospective and established clients, as these are major factors in determining the regulatory costs and timelines in Asian markets. R.O.S.E is the fast, convenient, and independent way to access this information directly for better planning, decision making, and professional development.
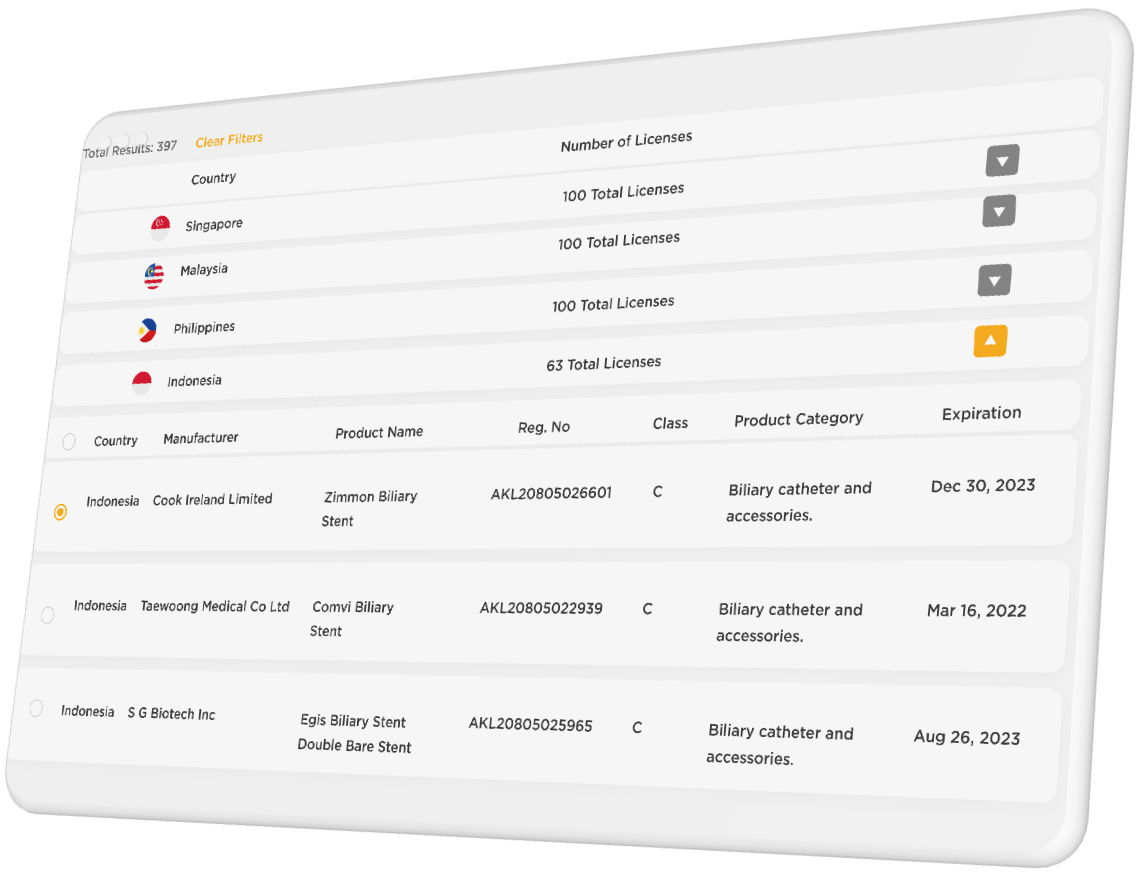
Search Results for ‘biliary stent’:
Founding R.O.S.E. members lock in a special introductory rate and will receive additional benefits including early access to new features and data. Coverage will begin with 4 countries in Southeast Asia with more countries added regularly to cover a total of 20 countries around the world. We intend to continuously improve the content, functionality, and utility of R.O.S.E. and encourage users to share feedback to help this service become the best it can be for regulatory professionals.
The R.O.S.E. platform is another step for Asia Actual in pursuing our goal to be the first choice for medical device manufacturers looking to grow their sales with a partner that provides clear, accurate guidance and support in Asian markets. Visit rose.asiaactual.com to start your free trial today.
Come Grow With Us
Asia Actual specializes in helping medical device manufacturers grow their sales in Asia and with experienced, bi-lingual commercial and regulatory experts on the ground in each market. Contact Asia Actual today with any questions or support requests.