Come grow with us in the US in Thailand in China in Korea in the Philippines in Taiwan in Hong Kong
Contact Us
US: +1 512 898-9222
SG: +65 3138-4148
EMAIL: Inquiry@asiaactual.com
Regulatory Spotlight: UDI Requirements in Singapore
Published on: May 14th, 2021
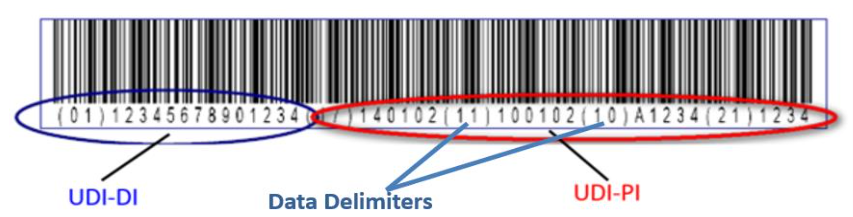
Singapore’s Health Science Authority (HSA) has announced that they will follow many countries example and begin implementing a Unique Device Identifier (UDI) system. UDI is an international system for identifying Medical Devices (MDs) meant to help quickly identify people affected by product recalls, device failures, or serious adverse events. UDI has been adopted by the International Medical Device Regulators Forum (IMDRF) to help harmonize global implementations and is in different stages of implementation around the world. UDI consists of a bar code and a numerical or alphanumerical system that quickly identifies the Device model, the Production information (batch/lot, software version, etc.), and Data delimiters.
UDI Requirements in Singapore
Singapore’s UDI requirements will align to internationally harmonized principles outlined in the UDI guidance published by the IMDRF.
- UDI guidance in 2013 (IMDRF/UDI WG/N7FINAL:2013)
- UDI Application Guide (IMDRF/UDI WG/N48 FINAL:2019)
The primary points of the new Singapore implementation include:
- UDI barcodes already implemented by manufacturers for EU and US is acceptable for devices to be supplied in Singapore. There is no need to create Singapore specific UDIs.
- UDIs of devices registered in Singapore can be viewed on the Singapore Medical Device Register (SMDR), which is public domain.
- Implementation timelines:
- 01 Jan 2022: Coronary stents, orthopedic joint replacement implants & Intraocular lens
- 01 Jan 2024: All other class D devices
- 01 Jan 2026: All class C devices
- 01 Jan 2028: All class B devices
- Class A devices do not need to have UDI, but manufacturers can implement it on a voluntary basis.
We are expecting HSA to further adjust the actual process and provide further updates, but the overall timelines for implementation remains unchanged.
Singapore’s HSA will also recognize the following organizations’ UDI Standards:
| Issuing Agencies | Link to UDI Example Labels |
| GS1 | Link |
| Health Industry Business Communications council (HIBCC) | Link |
| International Council for Commonality in Blood Banking Automation (ICCBBA) | Link |
UDI Requirements Around the World
Current UDI requirements are in different implementation stages around the world:
- USA: UDI has been implemented for all MDs (except some lower risk MDs)
- Phased implementation has started with high-risk MDs in 2014.
- EU: Implementation will start with high-risk MDs in 2021
- Australia: UDI requirements are under consultation
- Published for consultation in 2018 and 2019 respectively.
- China and Korea: Started UDI implementation process.
Components of UDI
UDI information includes a numeric or alphanumeric code that comprises of two parts: UDI-Device Identifier (UDI-DI) and UDI-Production Identifier (UDI-PI).
- Device Identifier (UDI-DI)
- A unique numeric or alphanumeric code specific to a model of medical device
- Mandatory, fixed portion of a UDI identifies a manufacturer’s specific product and package configuration
- Used as the “access key” to information stored in a UDI database (UDID)
- Production Identifier (UDI-PI)
- A numeric or alphanumeric code that identifies the unit of device production
- Includes serial number, lot/batch number, software version, and manufacturing and/or expiration date
- Data Delimiters
- Included in the Human Readable Information of the UDI to allow for legible interpretation of the coded information
- Different pre-determined Data Delimiters are used by different issuing agencies (e.g. GS1 – (01), (11) etc.; HIBCC -$, $$7 etc.; ICCBBA -=/, => etc.) UDI
Grow with Us
Asia Actual specializes in helping medical device manufacturers grow their sales in Asia with experienced, bi-lingual commercial and regulatory experts on the ground in Singapore. Contact Asia Actual today if you have questions or need support complying with the UDI requirements in Singapore.