Come grow with us in the US in Thailand in China in Korea in the Philippines in Taiwan in Hong Kong
Contact Us
US: +1 512 898-9222
SG: +65 3138-4148
EMAIL: Inquiry@asiaactual.com
Sources and Links
Sales Target Spotlight: China’s Boao Hope City
Published on: November 24, 2020
The Chinese national government has established a special economic zone on Hainan Island with a focus on healthcare. Medical device manufacturers seeking sales growth in China should be aware of the Boao Hope City development as a sales target, particularly for new, or innovative medical devices.
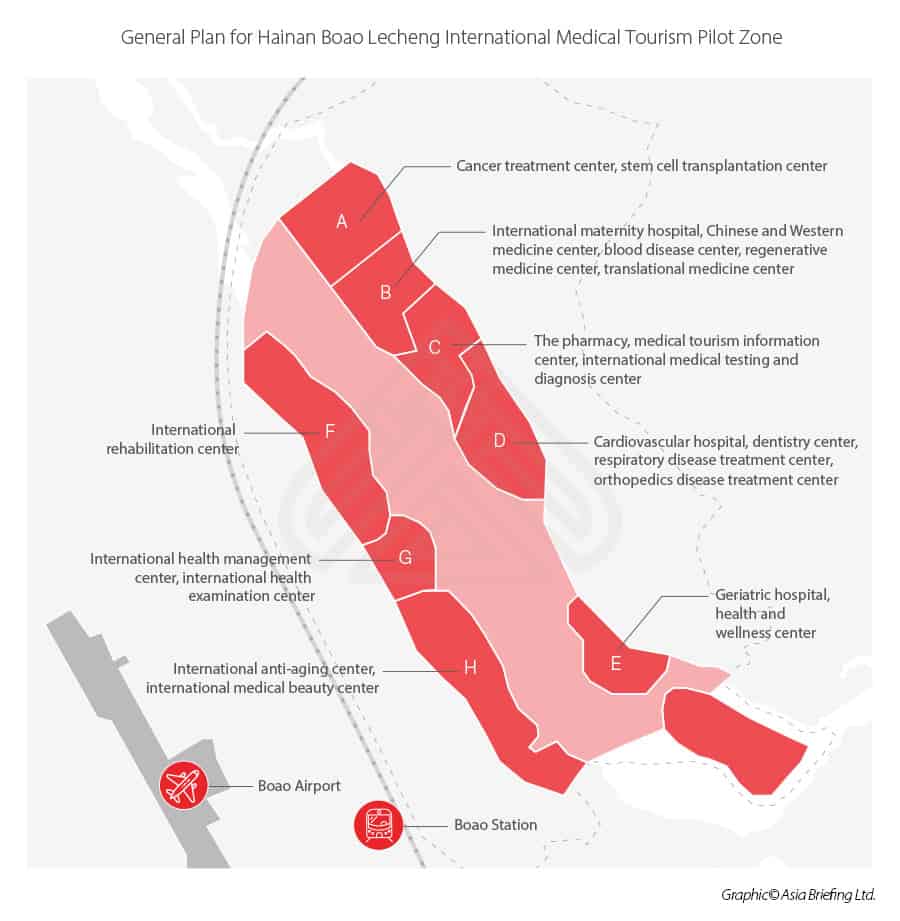
Located on the eastern side of Hainan island, Boao Hope City offers manufacturers many opportunities for sales growth and clinical research. Established in 2018, the Boao Lecheng International Medical Tourism Pilot Zone of Hainan Free Trade Port is a major initiative by the Chinese government to develop the province and continue taking steps towards trade reform. In addition to medical tourism, the Chinese Community Party (CCP) also intends on building a high-level free trade port with more open and free trade policies by 2025. Some of the advantages include visa free entry and easier access for foreign investors to Chinese companies and for Chinese companies and citizens to foreign companies. By 2030, the island is expected to be a world-renowned medical tourism destination with leading multinational companies conducting cutting edge research and treatments.
Medical Tourism on the Island
Boao Hope City now has 10 hospitals in operation with another 5 institutions expected to open by the end of 2020. Boao Hope City will initially provide wellness-oriented services including health management, rehabilitation, cosmetic surgeries, anti-aging treatments, and general research. One of the major features making this pilot zone appealing is how accommodating the regulations will be for foreign investors and visitors.
Below are some of the primary benefits Boao Hope City will offer compared to other regions in China:
- Expedited approval of Drugs and Medical Devices – Requires sponsorship from a local health professional and medical institution. Approved by local Hainan FDA office.
- Easy access for foreign professionals to come and go, making it more appealing for foreign investment and expert medical personnel.
- Allows patients to take specific drugs out of the pilot zone for personal use, even if they haven’t been approved in China.
- Increased cooperation with international organizations that gather real-world clinical data. The data can then be used to help expedite product approval for the mainland China population.
- Public Chinese hospitals will be able to take part in more private industry activities such as franchising, branding, and licensing technology to other institutions within the pilot zone.
- Development of new private insurance schemes to help pay for treatments and drugs.
- Develop a credit monitoring system for investors in the zone.
- Establish a post market surveillance system for evaluating and monitoring imported drugs and medical devices to facilitate clinical use and ensure public safety.
Free Trade Initiatives
Known as the Hawaii of China, Hainan province is located at the southern tip of China and is about the same size as Taiwan. Hainan has long been a popular tourist destination with 1.4 million foreign visitors in 2019 generating an estimated US$14.8 billion in economic activity. China plans to continue expanding the tourism industry as well as attracting foreign investment by converting the entire island to a Free Trade Zone (FTZ) and allowing unequaled access to foreign companies. For example, Qualified Foreign Limited Partner (QFLP) and Qualified Domestic Limited Partner (QDLP) entities are now permitted in Hainan province. These allow foreign investors the ability to create local investment funds and raise capital (domestically and internationally) that can then be used to invest in Chinese companies. Additionally, provisions for new insurance schemes, clinical trial cooperation, expedited registration routes for drugs and medical devices, combined with high-tech industries, tourism, and shopping, will make the island a very popular destination for investors and customers alike.
Foreign manufacturers should continue to watch how Boao Hope City evolves in the coming years as the free trade zone develops and the medical institutions on the island grow and expand into new specialties. Innovative companies can also consider Hainan as a potential steppingstone into the Chinese market by utilizing the real-world clinical data cooperation and gaining crucial clinical reputation before marketing to the greater Chinese market, and region in general.
Chinese Healthcare Statistics:
- It is estimated that more than 95% of China’s 1.3 billion citizens are covered by some level of health insurance. (Link)
- Health expenditure has soared from 500 billion yuan in 2000 to almost 6 trillion (US$907B) in 2018. (Link)
- An estimated 600,0000 mainland Chinese travel abroad each year seeking higher quality healthcare. This is largely due to the inability to find the products locally, primarily due to long and costly reviews, which can sometimes take up to 5 years. (Source: Hong Kong Trade Development Council (HKTDC))
- By 2050, China will have more than 330 million people over the age of 65. (https://time.com/5523805/china-aging-population-working-age/)
- In 2018, there were approximately 12,000 public hospitals and 21,000 private hospitals (excluding township hospitals and community hospitals. (National Health Commission, China Health Statistical Yearbook 2019.)
If you’d like to learn more about how your company can grow sales in Hainan, please contact Asia Actual today to schedule a free consultation.
Sales Call Spotlight is a series of blogs providing information on new or upcoming high-tech medical locations that make attractive partners for foreign manufacturers working in wellness, cosmetic surgery, regenerative medicine, cardiovascular, respiratory, dental, diagnosis, rehabilitation, anti-aging, and general hospital items.
Asia Actual is a regulatory consulting company specializing in helping manufacturers grow their sales through independent license holding, direct fulfillment, and a variety of sales channel support services.